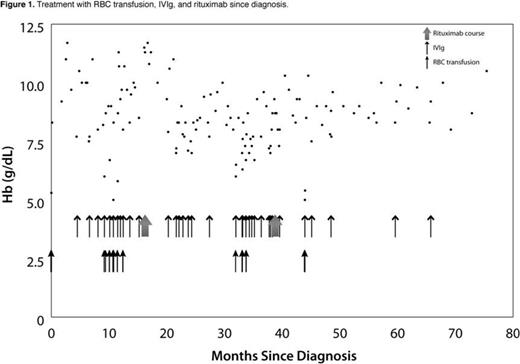
To determine the efficacy of rituximab in this condition, we searched the MEDLINE and EMBASE databases. Articles citing the use of rituximab to treat mixed-type or cold autoimmune hemolytic anemia (CAIHA) were identified. Ten studies (summarized in Table 1) and 43 case reports/series (48 total cases) met our criteria for inclusion. A complete response (CR) required Hb>12g/dL and the sustained absence of hemolysis. Disappearance of cold agglutinins was not required for a CR. A partial response (PR) required an improvement of symptoms, transfusion independency, and either: Hb>10g/dL or at least 2.0g/dL above pre-treatment levels, or a decrease in serum IgM concentration by at least 50%. Non-responders met neither CR nor PR criteria. Ages of the 26 male and 22 female patients ranged from 1-85 years with a median of 62 years. Diagnoses of CAIHA were reported in 40 cases, and mixed-type in 8. On average, patients had received 3 modes of treatment prior to rituximab. CRs were observed in 34 patients (70.8%), PRs in 7 (14.6%), and NRs in 7 (14.6%). Three of the 7 non-responders experienced an improvement in condition despite not meeting response criteria. Adverse effects were reported in only 3 cases and included a post-infusion low-grade fever, transient lymphopenia, and agranulocytosis (possibly due to CMV reactivation and not rituximab therapy).
Studies employing rituximab to treat cold or mixed autoimmune hemolytic anemia.
| Citation . | Study type . | Number of participants, Age range . | AIHA type . | Outcome (CR, PR, NR) . |
|---|---|---|---|---|
| Barcellini et al, 2012 | prospective multicenter | 9, 30-71 | cold | 16.6%, 33.3%, 0 |
| Berentsen et al, 2010 | prospective multicenter | 29, 39-87 | cold | 21%, 55%, 24% |
| Berentsen et al, 2001 | prospective | 6, 54-80 | cold | 14%, 57%, 29% |
| Berentsen et al, 2004 | prospective | 27, 51-91 | cold | 3%, 51%, 46% |
| Berentsen et al, 2006 | retrospective multicenter | 52, 51-96* | cold | single agent: 5%, 53%, 42% in combination: 25%, 42%, 33% |
| Cholankeril et al, 2012 | retrospective single institution | 6, 62-89 | 4 cold, 2 mixed | OR 100%, median Hb rise of 1.8g/dL |
| Peñalver et al, 2010 | retrospective multicenter | 9, 20-86* | cold | CR and MR 66.7% |
| Schöllkopf et al, 2006 | prospective multicenter | 20, 54-86 | cold | 5%, 40%, 55% |
| Dierickx et al, 2009 | retrospective multicenter | 14, 1-87* | cold | 28.6%, 35.7%, 35.7% |
| Zecca et al, 2003 | prospective | 1, 1.3 | cold | responder |
| Zaja et al, 2003 | not specified | 1, 72 | cold | complete responder |
| Citation . | Study type . | Number of participants, Age range . | AIHA type . | Outcome (CR, PR, NR) . |
|---|---|---|---|---|
| Barcellini et al, 2012 | prospective multicenter | 9, 30-71 | cold | 16.6%, 33.3%, 0 |
| Berentsen et al, 2010 | prospective multicenter | 29, 39-87 | cold | 21%, 55%, 24% |
| Berentsen et al, 2001 | prospective | 6, 54-80 | cold | 14%, 57%, 29% |
| Berentsen et al, 2004 | prospective | 27, 51-91 | cold | 3%, 51%, 46% |
| Berentsen et al, 2006 | retrospective multicenter | 52, 51-96* | cold | single agent: 5%, 53%, 42% in combination: 25%, 42%, 33% |
| Cholankeril et al, 2012 | retrospective single institution | 6, 62-89 | 4 cold, 2 mixed | OR 100%, median Hb rise of 1.8g/dL |
| Peñalver et al, 2010 | retrospective multicenter | 9, 20-86* | cold | CR and MR 66.7% |
| Schöllkopf et al, 2006 | prospective multicenter | 20, 54-86 | cold | 5%, 40%, 55% |
| Dierickx et al, 2009 | retrospective multicenter | 14, 1-87* | cold | 28.6%, 35.7%, 35.7% |
| Zecca et al, 2003 | prospective | 1, 1.3 | cold | responder |
| Zaja et al, 2003 | not specified | 1, 72 | cold | complete responder |
*age range reflects entire study population, not CAIHA subgroup
OR=overall response, MR=maintained response
In summary, we report a case of CAIHA. A literature summary supported the efficacy of rituximab for this condition. Based on our observations we recommend the use of rituximab over alternative therapies for patients with CAIHA to reduce symptoms, need for transfusion and reduce exposure to immunosuppressive drugs (Grade 2C Recommendation).
Off Label Use: Rituximab was used to treat cold autoimmune hemolytic anemia. Crowther:Asahi Kasai: Membership on an entity’s Board of Directors or advisory committees; Baxter: Membership on an entity’s Board of Directors or advisory committees, Speakers Bureau; Boehringer Ingelheim: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees; CSL Behring: Speakers Bureau; Leo Pharma: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding, Speakers Bureau; Merck: Consultancy; Octapharma: Consultancy, Membership on an entity’s Board of Directors or advisory committees; Pfizer: Consultancy, Honoraria, Research Funding; Sanofi-Aventis: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Viropharma: Membership on an entity’s Board of Directors or advisory committees.