Children with acute, severe cardiopulmonary compromise may require support with ECMO. Adequate anticoagulation without inducing life-threatening hemorrhage plays a critical role in the management of these patients. Recent data from adult studies suggest that patients undergoing cardiac bypass for coronary artery bypass grafting (CABG) often have reduced Factor XIII levels (FXIII). This relative FXIII deficiency may be predictive of hemorrhage or severe blood loss. Such studies have never been conducted in the pediatric population. Our primary objective will be to determine if a relative deficiency in FXIII occurs in children undergoing ECMO and to determine any time dependency of these changes. Our secondary objective will be to determine if blood loss and/or transfusion requirements during ECMO correlates with FXIII levels.
Single center ongoing study utilizing a prospective design involving pediatric patients (ages 0-18) undergoing ECMO. Samples for FXIII, D-Dimers, Fibrinogen and Thrombin Time are being drawn at designated time points as follows: prior to or during ECMO cannulation, 2 hours, 24 hours, 3-5 days and 14-21 days after initiation of ECMO and 1-3 days after discontinuation of ECMO. Mixed effects analysis of covariance (ANCOVA) will be used for data analysis.
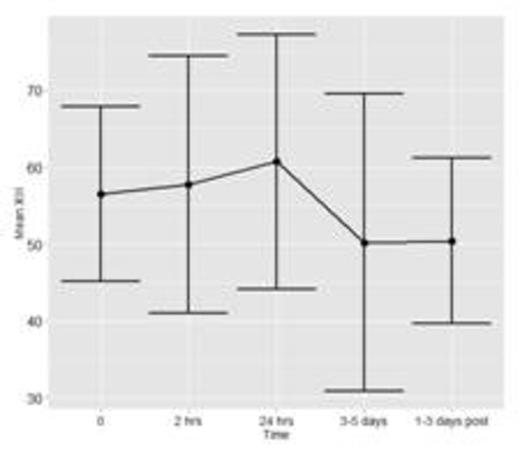
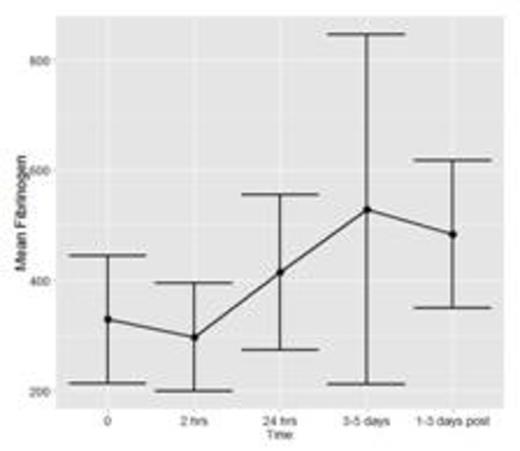
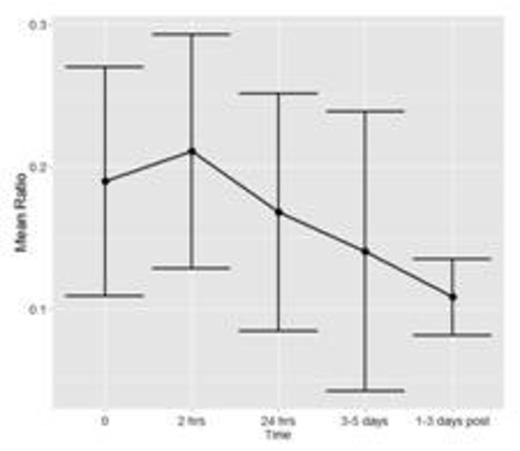
To date, fifteen patients have been enrolled and analyses have been conducted for n=7 patients with a total of 36 time points of data. Figure 1a and 1b show the mean and standard deviation (SD) of FXIII and Fibrinogen, respectively. Figure 1c shows the mean ratio of FXIII to Fibrinogen. Error bars represent 1 SD around the mean (central point).
This is the first pediatric study evaluating FXIII activity in children undergoing ECMO. Firm conclusions cannot be drawn with the interim analyses conducted to date. If variations in FXIII are found, our results will provide important information towards determining if children undergoing ECMO could potentially benefit from FXIII replacement, in turn minimizing bleeding complications and/or transfusion requirements often encountered with this treatment modality.
No relevant conflicts of interest to declare.