Abstract
In 2007, a battery powered bone marrow aspiration and biopsy system (OnControl, Vidacare Corp, Shavano Park, TX) was developed and cleared by the US FDA for adult patients. Multiple studies have evaluated the use of the Powered device in adults and found decreased procedure time, decreased patient pain, improved core biopsy specimens and a higher degree of operator satisfaction compared to traditional Manual Jamshidi-type devices; but there have been no studies demonstrating the system's utility for pediatric patients. In a study sponsored by Vidacare, we conducted a randomized controlled trial to compare the Powered bone marrow aspiration and biopsy system to Manual devices in children.
A randomized controlled trial was developed to enroll a total of 44 patients between ages 2 and 18 years old requiring a bone marrow evaluation. Patients were assigned to have a single procedure carried out using the Powered device or the Manual device. Data were also collected for patients needing bilateral marrow evaluations for direct comparison between the two devices. The time to obtain the aspirate and biopsy were recorded, as was the pain score post-procedure. A blinded pathologist recorded the length, width, and overall quality of the specimen. Operators performing the procedure also completed a survey to evaluate the safety of the device, and their satisfaction with the device.
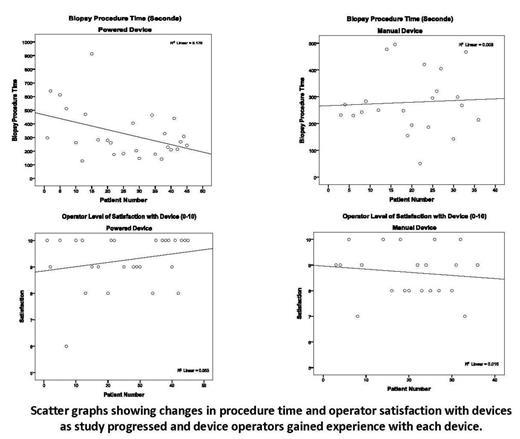
Forty-four patients were enrolled in the study and 5 patients received bilateral procedures; for a total of 49 procedures (Powered n=27, Manual n=22). The mean age was 9.8 (±0.8) years and 60% were male. Statistically, there was no difference in device performance in terms of procedure time, patient pain, operator satisfaction, specimen quality or specimen size. There was a significant difference in perceived device safety and specimen width in favor of the Powered device (p=.034 and p=.027, respectively); and a trend toward significance in terms of operator satisfaction, favoring the Powered device (p=.060). For the Powered device, but not the Manual device, procedure times improved with continued experience, as did biopsy core length and operator satisfaction. There was a significant correlation between level of experience and procedure time. No significant complications were observed using either device. Operators commented that the Powered device was useful when performing procedures on older patients and those with blast-packed marrows.
In children, the Powered marrow device may provide benefit over Manual methods as reported in previous adult studies. We found that the OnControl™ biopsies were obtained with a high degree of safety, with good quality and size, and with a high degree of operator satisfaction. Such benefits may lead to reductions in anesthesia time and overall costs. Over time and with more procedures, operators performing procedures noted increased satisfaction and better performance with the Powered device than they did with the Manual device, in both clinical use and ease of training future providers.
Falcon-Cantrill: Vidacare Corporation: Research Funding. Off Label Use: Study device (OnControl)is cleared for adult patients, but our study using the device involved pediatric patients. Thomas:Vidacare Corporation: Research Funding. Saldivar:Vidacare Corporation: Research Funding. Philbeck:Vidacare Corporation: Employment. Assanasen:Vidacare Corporation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.