Abstract
Relapsed/refractory AML patients have a poor prognosis; allogeneic hematopoietic stem cell transplantation (HSCT) is the only chance in this setting to achieve long-term disease-free survival (1). It was previously established the activity of clofarabine plus cytarabine in AML relapse (clofarabine dosed once daily for 5 days with 40 mg/m2 followed 4 hours later by ara-C at 1 g/m2 per day)(2).However, modifications of this combination in AML therapy of relapsed/refractory patients warrant further evaluation. Therefore, our goal was to determine the efficacy and safety of clofarabine at lower dosage followed by cytarabine (Ara-C) in adult patients with relapsed or refractory acute myeloid leukemia (AML) and to evaluate the capacity of this regimen as a bridge for HSCT.
Patients aged 18-65 years with refractory/relapsed AML were treated at the dose of clofarabine 30 mg/mq on days 1-5 and cytarabine 1000 mg/mq gg on days 1-5. We evaluated the complete remission rate (CRR), duration of remission (DOR) and overall survival (OS). Minimal residual disease (MRD) by molecular targeting was considered in all patients.
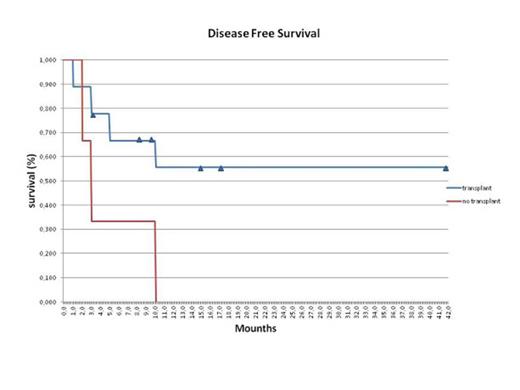
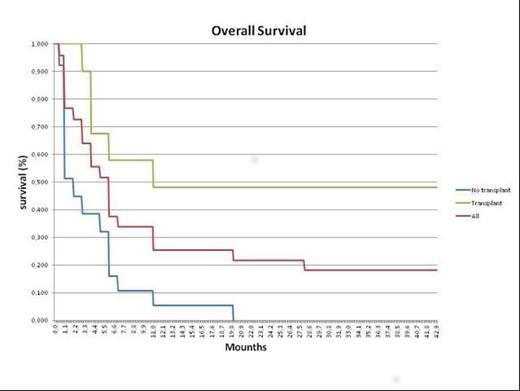
Twenty-five (25) patients aged 29-64 years (median 47), who were fit for allogenetic HCT, received one cycle of 30 minutes infusion of clofarabine 30 mg/mq, followed 4 hours later by 3 hours infusion of intermediate dose cytarabine 1000 mg/mq days 1-5. Only in the first three patients this schedule was followed by gentuzumab. Nine (36%) patients had refractory disease (seven after one induction regimen, one after two previous regimes, one after a prior hematopoietic stem cell transplant (HSCT); 16 (64%) patients were in their first (12 patients) or second relapse (4 patients); among the 12 patients in first relapse, 5 were from an allogeneic stem cell transplant. Fourteen patients (56%) achieved a complete remission (CR), seven (28%) was refractory and 4 (16%) died of treatment related mortality. Eleven (44%) patients underwent (9 in CR) to allogeneic transplants or DLI infusion (3 patients refractory, and 8 patients relapsed), only one patient underwent to autologous transplant. One patient, who was relapsed after prior HSCT, obtained a CR but he developed acute graft vs host disease after therapy and died in molecular CR*. Among all patients underwent HSCT after Clofa/Ara-c salvage, six patients (50%) are still alive and in complete remission, six patients (50%) died because of HSCT complications or AML relapse. The complete remission rate (CRR) was (56,00 %), the median Overall Survival was 5 months for all patients (range 1-38 M), 11 Months for those underwent to tranplantation and 1,5 Months for non transplanted group. Treatment was complicated by neutropenic fever (n=17), grade III-IV mucositis (n=2) , skin rush (n=4) grade II- III, hepatic transaminase elevations (n=2). Two (n=5) patient died before their disease status could be evaluated.
These preliminary results suggest that combination treatment with clofarabine 30 mg/mq and ARA-C 1000 mg/mq is effective in this particularly poor prognosis category of patients, resulting in an ORR very favorably, representing a potential “bridge” toward bone marrow transplant procedures (among the 14 patients who achieved a CR, twelve (85%) proceeded to HSCT, and six are still alive). The safety profile is acceptable in this relapsed/refractory population, and our results are very similar to previous regimes using higher clofarabine dosages. More studies with this combination in adults are warranted.
1 Estey E. Treatment of relapsed and refractory acute myeloid leukemia. Leukemia. 2000;14:476-479. 2. Faderl S et al, “Results of a pase 1-2 study of clofarabine in combination with cytarabine (ara-C)”Blood 2005
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.