Abstract
Dramatic changes in overall survival of patients (pts) with chronic myeloid leukemia (CML) in chronic phase (CP) have occurred since tyrosine kinase inhibitors (TKIs) were implemented in the treatment strategy. But there are still many issues in therapy of advanced phase disease, especially in blastic phase (BP). Allogeneic stem cell transplantation (alloSCT) is still the only curative option for CML BP, so all efforts should be focused on bringing pts to alloSCT. Thus optimal approach to obtain at least stable hematologic response before alloSCT is needed.
Since 2008, 14 pts (4 more pts with isolated extramedullary BP were not included) with CML BP were admitted to our clinic. These were 8 males and 6 females with a median age of 44 years (range; 21-63) at the time of BP. The types of BP were: biphenotypic (n=1), undifferentiated (n=1), myeloid (n=8) and lymphoid (n=4). All pts except 2 (1 with BP and 1 with accelerated phase) were initially diagnosed as CP. Median time from diagnosis to BP was 37 months (range; 0-83). Before BP all pts except 2 were pretreated with imatinib and 6 of them, after failing imatinib, received one or more new TKIs. First line therapy in BP was monotherapy with new TKI (n=5) or chemotherapy (“7+3”, “RACOP”, low doses of Ara-C, “Hyper-CVAD”, “Dexa+VCR”) with or w/o TKI (n=9). Responses are specified in table 1.
Efficacy of monotherapy with TKI or chemotherapy with or w/o TKI in BP CML.
| . | Mono TKI (n=5) . | Chemotherapy with or w/o TKI (n=9) . |
|---|---|---|
| Overall HR (returm to CP or complete HR) | 3 | 6 |
| CCyR**+MMR*** | 1 | 1 |
| No response | 1 | 2 |
| Duration of the best response, median (range) | 3,5 months (0,5-9) | 11 months (1,5-97) |
| FLAG therapy, n= | 2 | 3 |
| Allo SCT, n= | 2 (n=1 after FLAG) | 3 (n=1 after FLAG) |
| Alive, n= | 3 (n=2 after alloSCT, n=1 after FLAG - pending alloSCT) | 4 (n=2 after alloSCT, n=2 after FLAG) |
| . | Mono TKI (n=5) . | Chemotherapy with or w/o TKI (n=9) . |
|---|---|---|
| Overall HR (returm to CP or complete HR) | 3 | 6 |
| CCyR**+MMR*** | 1 | 1 |
| No response | 1 | 2 |
| Duration of the best response, median (range) | 3,5 months (0,5-9) | 11 months (1,5-97) |
| FLAG therapy, n= | 2 | 3 |
| Allo SCT, n= | 2 (n=1 after FLAG) | 3 (n=1 after FLAG) |
| Alive, n= | 3 (n=2 after alloSCT, n=1 after FLAG - pending alloSCT) | 4 (n=2 after alloSCT, n=2 after FLAG) |
HR-Hematologic response; CCyR-complete cytogenetic response; MMR-major molecular response; alloSCT-allogeneic stem cell transplantation.
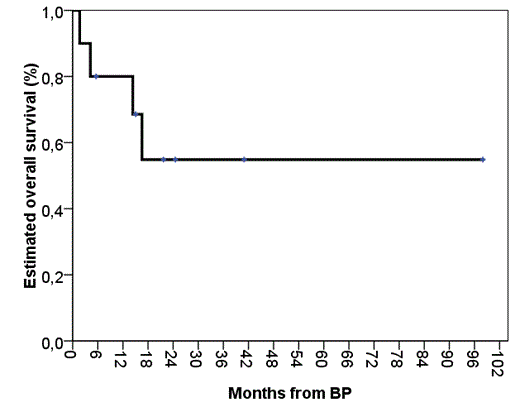
FLAG regimen was subsequently given to 5 pts as second or more line therapy after failure of previous monoTKI (n=1) or Rx + TKI (n=4). Median time from BP to FLAG was 3,5 months (range; 1,5-21). The best response to FLAG therapy was complete hematologic (n=1), complete cytogenetic with (n=1) or w/o (n=1) major molecular response. There were no responses in 2 cases. All responders maintain their response after median follow up (FU) of 2 months (range; 1,5-5). All patients treated with FLAG are alive (2 after alloSCT, 3 pending alloSCT). Only 1 ptn reached alloSCT w/o any Rx after monoTKI. AlloSCT was successful in 4/5. Median FU time for patients alive after alloSCT is 12 months (range; 3,5-25). For whole group after a median FU of 14 months, 7/14 (50%) pts are alive, including 4 pts after alloSCT. Estimated 3-year overall survival for all pts is 54% (fig. 1).
Estimated 3-year overall survival for all pts.
All CML BP patients treated with TKIs alone lost their response in a short time. Responses were much more durable in pts treated with Rx +/- TKIs. FLAG regimen was effective even in pts with failure to previous Rx+TKIs. The majority of pts after alloSCT are alive. Chemotherapy, including FLAG with concomitant or subsequent TKIs, had advantage over monoTKI both in overall and progression free survival in CML BP.
Lomaia:Novartis: Honoraria, Travel grants Other; Bristol-Myers Squibb: Honoraria, Travel grants, Travel grants Other. Zaritskey:University of Heidelberg: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.