Abstract
Chronic lymphocytic leukemia (CLL) can effect renal function in a variety of ways including direct infiltration of the kidney, ureteral obstruction by lymphadenopathy, and treatment related tumor lysis syndrome (uric acid nephropathy). Rarely, CLL has also been reported to be associated with light chain nephropathy, renal amyloidosis, membranoproliferative glomerulonephritis (MPGN), granulomatous interstitial nephritis (GIN), and minimal change disease (MCD). Nearly all the data on the effects of CLL on renal function is at the case report level. We systematically evaluated the prevalence of renal insufficiency at diagnosis as well the incidence of acquired renal insufficiency during follow-up in a large cohort of patients with newly diagnosed CLL to more accurately define the effects of CLL on the kidney and its impact on clinical outcomes.
Between January 1995 -February 2013, previously untreated CLL patients seen in the Division of Hematology at Mayo Clinic at diagnosis (<12 months) and who had baseline assessment of serum creatinine were included in this analysis. Patients with serum creatinine (Cr) ≥1.5 mg/dL at baseline were classified as having renal insufficiency at diagnosis. Patients who initially had baseline creatinine <1.5 mg/dL but who developed a Cr≥1.5 mg/dL during the course of their disease were considered to have acquired renal insufficiency.
Existing renal insufficiency at the time of CLL diagnosis:
Of 2047 patients who met the eligibility criteria, 153 (7.5%) patients had renal insufficiency (Cr≥1.5 mg/dL) at the time of CLL diagnosis including 15 (0.7%) with a Cr≥3 mg/dL. Renal insufficiency was also more common among men (9.3% vs. 3.9%; p<0.00001), those with advanced stage disease (Rai 0=7.0%; Rai I-II=6.4%, Rai III-IV=20.2%; p<0.0001), and CD49d positive patients (6.8% vs. 3.8%; p<0.038). Patients with renal insufficiency at diagnosis were also older (median age 72.2 vs. 63.9; p<0.0001). No difference in the prevalence of renal insufficiency at diagnosis was observed based on cytogenetic abnormalities detected by FISH or CD38, ZAP-70 or IGHV gene mutation status. Although renal insufficiency at diagnosis was strongly associated with OS on univariate analysis (p<0.001), no association was observed between renal insufficiency and TTT or OS on multi-variate analysis adjusting for age, sex, and Rai stage.
Acquired renal insufficiency during CLL disease course:
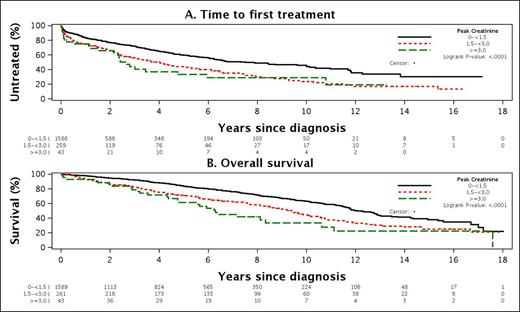
Among the 1894 patients with normal renal function at diagnosis, 304 (16.1%) acquired renal insufficiency (Cr≥1.5 mg/dL) during the course of their CLL disease course including 43 (2.3%) with peak Cr≥3 mg/dL. In addition to age (older) and male sex, a number of CLL disease characteristics were associated with a higher likelihood of acquired renal insufficiency including: IGHV UM (OR=2.0; p=0.0001), unfavorable FISH (del17p- or 11q-; OR=2.0; p=0.001), and being CD49d+ (OR=1.8; p=0.002), ZAP-70+ (OR=1.6; p=0.004), or CD38+ (OR=1.4; p=0.0.032),. Shorter TTT (p<0.001) and OS (P<0.001) was observed among patients with initially normal creatinine who acquired renal insufficiency (Figure 1A and 1B). On MV analysis adjusting for age, sex, and stage at diagnosis, acquired renal insufficiency remained an independent predictor of TTT (OR=1.77; p=0.001) and OS (OR=2.67; p<0.001).
Renal insufficiency and therapy selection
After median follow-up of 4.5 years (range 0-18.0), 620 of 2047 (30.3%) patients have progressed to require treatment. Patients with renal insufficiency prior to treatment were less likely to receive purine nucleoside analogue based therapy and more likely to receive single agent alkylator based treatment.
Approximately 1 in every 13 patients (7.5%) with CLL has renal insufficiency at the time of diagnosis and an additional 16.1% acquire renal insufficiency during the course of the disease. The risk of developing renal insufficiency is associated with a variety of CLL B-cell characteristics and is associated with TTT and OS. Data on causes of acquired renal insufficiency is being abstracted and will be presented at the meeting.
Time to first treatment (A) and Overall Survival (B) among patients with initially normal creatinine who acquired renal insufficiency during the course of follow-up
Time to first treatment (A) and Overall Survival (B) among patients with initially normal creatinine who acquired renal insufficiency during the course of follow-up
Shanafelt:Genentech: Research Funding; Glaxo-Smith-Kline: Research Funding; Cephalon: Research Funding; Hospira: Research Funding; Celgene: Research Funding; Polyphenon E International: Research Funding. Off Label Use: MK2206 in a phase 1 trial of CLL.
Author notes
Asterisk with author names denotes non-ASH members.