Abstract
The aim of this study is to identify risk factors associated with the development of severe bacterial infection (SBI) in patient with multiple myeloma (MM) during treatment with bortezomib-containing regimens.
A total of 98 patients with MM who were treated with bortezomib-based treatment between 2006 and 2012 were analyzed. Fifty three patients received bortezomib-dexamethasone, 25 patients received bortezomib-melphalan-prednisolone, 15 patients received bortezomib-doxorubicin-dexamethasone and 5 patients received other regimens. They received a total of 427 courses of treatment. The SBI was defined as at least grade 3 neutropenic/non-neutropenic infection by NCI Common Terminology Criteria for Adverse Events version 4.0. Using the logistic regression method, we analyzed risk factors for the development of SBI during each course of treatment.
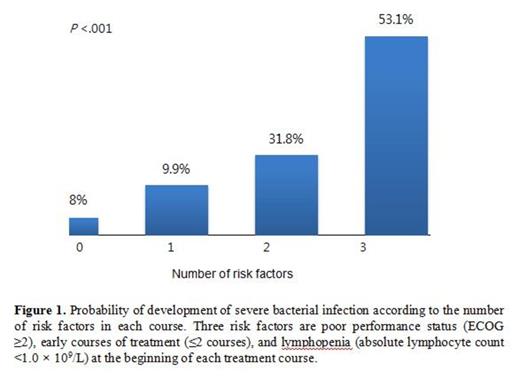
Median age of the patients was 62 years and 40.6% (30/98) of patients treated with bortezomib-containing regimens as first-line therapy. The SBI was developed in 57% (56/98) of patients and 19% (81/427) of bortezomib courses. Among 81 episodes of SBI, 42 (53%) episodes were clinically documented infection, 30 (37%) were microbiologically documented infections, and 9 (11%) were fever of unknown origin. The most common type of infection was pneumonia (60%). Poor performance status (ECOG ≥2) (Hazard Ratio [HR] 5.365, 95% Confidence Interval [CI] 2.004-14.364, P =.001) was the only risk factor for the development of SBI in 98 patients. When we analyzed the risk factors for the development of SBI which occurred during each treatment course, poor performance status (ECOG ≥2) (P <.001), early course of treatment (≤2 courses) (P <.001) and pretreatment lymphopenia (absolute lymphocyte count <1.0 x 109/L) (P = .043) were associated with increased risk for developing SBI in each course. These 3 risk factors remained independently significant in multivariate analysis: poor performance status (ECOG ≥2) (HR 3.920, 95% CI 2.305-6.666, P <.001), early course of treatment (≤2 courses) (HR 2.782, 95% CI 1.633-4.740, P <.001) and pretreatment lymphopenia (HR 1.728, 95% CI 1.016-2.937, P = .043). The probability of developing SBI in each treatment course was 5.1% in courses with no risk factor, 14.9% in 1 risk factor, 23.9% in 2 risk factors and 59.5% in 3 risk factors (P <.001, Figure 1 ). After treatment with bortezomib-containing regimens, patients who experienced SBI showed a significantly shorter overall survival than patients who didn't experienced SBI (median 6.1 month vs. 30.1 months, P = .004) although progression free survival was not different (median 18.1 months vs. 21.9 months, P = .418). The multivariate cox analysis demonstrated that the development of SBI was associated with inferior overall survival (HR 2.440, 95% CI 1.305-4.561, P = .005) as well as male sex (HR 2.323, 95% CI 1.236-4.367, P = .009).
In conclusion, we identified that poor performance status, early courses of treatment, and lymphopenia at the beginning of each treatment course were the risk factors for the development of SBI in patients with MM during treatment with bortezomib-containing regimens. Close monitoring for the development of SBI and appropriate treatment should be considered in the patients with risk factors.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.