Abstract
Relapse is the major cause of death after allogeneic hematopoietic cell transplantation (HCT) for hematological malignancies and can be treated with donor lymphocyte infusions (DLI). Initial low dose DLI followed by escalating DLI doses have been used to minimize the risk of graft-versus-host disease (GVHD) in patients with relapsed chronic myeloid leukemia (CML). The impact of initial CD3+ cell dose on outcome after DLI in patients treated for other hematological malignancies is limited. We retrospectively analyzed data of 75 patients who were treated with DLI after HCT at our institution over the last 20 years. The primary objective of this analysis was to determine the effect of the initial CD3+ cell dose in DLI on overall survival (OS).
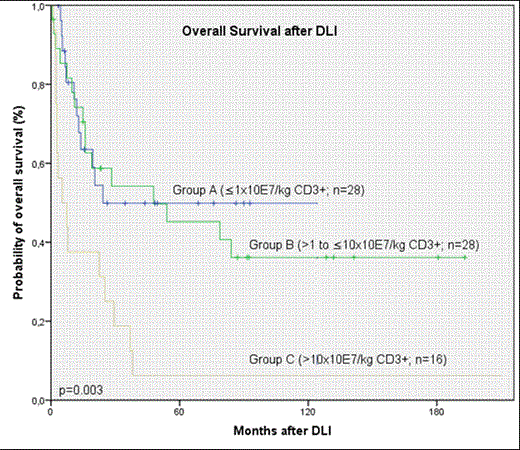
The median age of the cohort was 43 (range, 18 - 71) years, 60% were male. Patients received transplants from related (n=53) or unrelated (n=22) donors for treatment of CML (n=18), acute myeloid leukemia (n=29), myelodysplastic syndrome (n=3), acute lymphoblastic leukemia (n=9), multiple myeloma (n=8), lymphoma (n=7) and paroxysmal nocturnal hemoglobinuria (n=1). Thirty-three patients (44%) received reduced-intensity conditioning regimens. DLI were given for mixed chimerism (n=5), relapse/progression (n=58) or prophylactically in patients with high risk disease (n=12). The median time intervals from HCT to relapse/progression and HCT to DLI for mixed chimerism were 15 (range, 1-80) and 20 (range, 1-169) months, retrospectively. Prophylactic DLI were given between 1 and 25 months after HCT. Therapy prior to DLI consisted of cytoreductive chemo-/radiotherapy with or without tyrosine kinase inhibitors (TKI) in 33 (44%) patients, TKIs alone in 10 (13%) and immunomodulatory drugs in 3 (4%) patients. Twenty-nine (39%) patients received DLI alone. Initial DLI CD3+ cell dose/kg was <1x107 (n=28; Group A), >1.0 to <10x107 (n=28; Group B), and >10x107(n=16; Group C), in 3 patients the cell dose was not available. Of note, 24 patients received escalating doses of CD3+ cells.
Response evaluation three months after DLI for evaluable patients with relapse/progression or mixed chimerism (n=60) revealed complete response (CR) in 30 (47%) and no response in 28 (44%) patients. Two patients developed aplasia and were rescued with second HCT. After prophylactic DLI 6/12 (50%) patients remained in CR whereas 6 (50%) experienced relapse between 3 and 21 months after DLI. Twenty-nine (39%) patients are currently alive including eight with malignant disease after a median follow-up of 69 months (range, 1-193). Seventeen (24%) patients developed acute or chronic GVHD after DLI. The risk for GVHD was significantly higher in patients receiving higher (Group B and C) compared to lower doses (Group A; p=0.04) of CD3+. Eight patients died due to severe GVHD. Higher CD3 cell doses did not result in lower relapse rates or better response (p=0.23). OS rates at 5 years were 50%, 45%, and 6% for Groups A, B, and C, respectively. Compared to lower doses initial DLI CD3+ cell dose of >10x107/kg was significantly associated with a reduced overall survival after DLI (p=0.003, Figure 1). Of note, conditioning regimen applied, donor type and reason for DLI administration had no significant influence on overall survival.
Our results show that an initial DLI CD3+ cell dose of >10x107/kg is associated with reduced survival rates. These findings are clinically relevant, and support a recommendation to infuse <10x107 CD3+ cells/kg for treatment of hematological malignancies after HCT. Additionally, molecular monitoring of patients is warranted to allow the use of DLI early in the course of their disease.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.