Abstract
The bone marrow (BM) microenvironment is characterized by hypoxia and the presence of supporting mesenchymal stromal cells (MSC) that promote leukemia cell survival and resistance to therapy, in part by metabolic reprogramming. However mechanisms that couple leukemic cells survival to metabolic processes under different microenvironment conditions have not been elucidated. Glutamine (Gln) provides cells with carbon skeletons to the Krebs cycle (KC) via anaplerosis, sustains cell proliferation, regulates redox homeostasis and modulates activity of signal transduction pathways. Recent data suggests that leukemia cells reduce molecular oxygen utilizing electrons from carbon sources other than pyruvate, and we hypothesize that these electrons could be provided at least in part by glutaminolysis. Our recent studies utilizing gene expression profiling indicate that MSC co-culture under hypoxia promoted glycolytic gene expression in AML cells, as well as genes regulating oxidative phosphorylation (OXPHOS), KC cycle and Gln utilization (GLS1, GOT) (Matre et al., AACR 2013:1887). Here we report studies aimed to unravel metabolic changes in proliferating leukemic cells under hypoxia and upon interaction with MSC and determine the role of Gln as a contributor.
First, we performed GC-MS metabolic profiling of OCI-AML3 leukemic cells alone or in co-cultured with MSC under hypoxic or normoxic conditions and observed significant changes in the core metabolic processes. Our data demonstrates that microenvironment promotes glucose-independent OXPHOS to meet bioenergetics needs of leukemic cells. Interaction with MSC propels a glucose-independent oxidative KC through Gln and asparagine catabolism even under conditions where oxygen concentration is limited. Under hypoxia, concentrations of KC intermediates were lower compared to normoxia, however the accumulation of 2-hydroxyglutarate suggests reverse KC activity with glutamate-derived 2-oxoglutarate being converted to citrate via reductive carboxylation pathway. In addition, consumption of glucogenic amino acids was upregulated by MSCs. Glycolytic intermediates accumulated under hypoxia and coculture accompanied by excretion of pyruvate as lactate, suggesting increased availability of carbon skeletons for biomass generation provided, in part, by glutaminolysis.
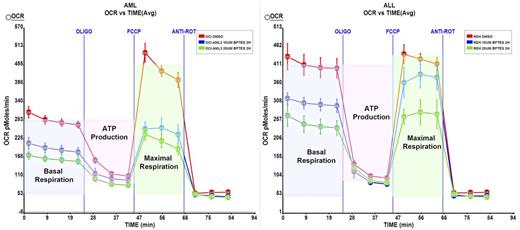
Analysis of a panel of acute leukemia cell lines (n=12) showed that subset of leukemia (75%) markedly dependent on Gln for growth with Gln deprivation causing steep decrease in viable cell number via induction of apoptosis. In addition, in the corresponding subset, inhibition of GLS (GLS1) with BPTES decreased cell growth and increased apoptosis under both normoxia and hypoxia. Notably, MSC co-culture failed to protect firmly attached hypoxic AML cells, which are otherwise resistant to chemotherapy-induced cytotoxicity. Finally, the expression of GLS1 gene splice variants, Glutaminase C (GAC) and kidney glutaminase (KGA), was determined using oligonucleotide microarrays (HG U133 Plus 2.0, Affymetrix) in 288 AML and in 103 normal samples (healthy BM and non-leukemia conditions, Haferlach, JCO 2010). GAC transcript was found to be significantly overexpressed in several AML subtypes, including AML with FLT3 gene mutations and complex cytogenetics. In turn, KGA expression was not different between AML and normal samples.
In summary, our results indicate that Gln is a major source of carbon skeletons for KC activity in AML cells, and demonstrate the key role of Gln utilization pathway for the survival of hypoxic BM-resident leukemic cells and “Glutamine-dependent OXPHOS subset” of leukemia. These findings support the notion of targeting microenvironment-fueled leukemia metabolism through pharmacological inhibition of GLS with novel selective GLS1/2 inhibitors entering clinical arena.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.