Abstract
The JAK1/JAK2 inhibitor ruxolitinib (RUX) is approved for treatment of myelofibrosis (MF). However, some patients do not respond to RUX, while others lose responsiveness or develop intolerance to RUX over time. Preclinical data also suggest that certain JAK2 mutations (eg the gatekeeper mutation M929I) may confer resistance to RUX (Leukemia 2012;26:708). Options for these RUX resistant/intolerant patients, particularly with regard to further JAK inhibitor therapy, are unclear. Fedratinib (SAR302503) is a JAK2-selective inhibitor which has demonstrated clinically meaningful reductions in splenomegaly and symptom burden in patients with MF, with an acceptable and consistent safety profile (J Clin Oncol 2011;29:789. Haematologica 2013;98:S1113). Fedratinib sensitivity in vitro is unaffected by the M929I mutation. This pre-specified interim analysis of the JAKARTA-2 study (NCT01523171) investigated the efficacy and safety of fedratinib in MF patients previously treated with RUX.
JAKARTA-2 is a Phase II, multicenter, open-label, single-arm study of patients who previously received RUX for MF. Patients ≥18 yrs with splenomegaly and platelet count ≥50 ×109/L received fedratinib 400 mg orally, once daily for consecutive 4-wk cycles (permitted dose adjustment to 200–600 mg daily). Eligible patients had received ≥14 d RUX treatment, and had discontinued RUX for ≥14 d prior to starting fedratinib. Although there is no consensus definition of RUX resistance/intolerance, for the purpose of this study patients were classed (investigator assessment) as resistant (lack or loss of response) or intolerant to RUX. Primary endpoint for the interim analysis: spleen response rate (RR) (≥35% reduction in spleen volume from baseline at Wk 12 [MRI/CT blinded central review]) in the per-protocol (PP) population. Secondary endpoints: symptom RR (≥50% reduction in total symptom score [TSS: modified Myelofibrosis Symptom Assessment Form] from baseline to Wk 12) and safety.
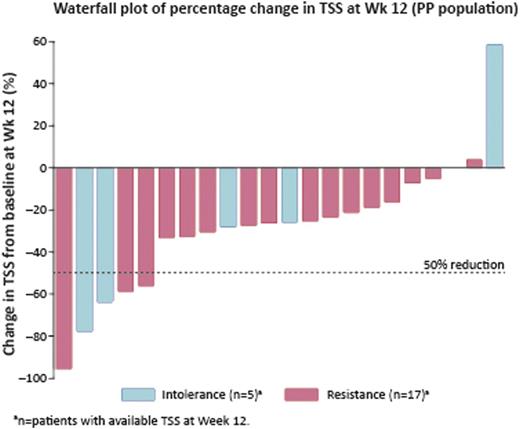
At the cut-off date for this interim analysis, 27 patients had received fedratinib treatment (median RUX exposure 10.7 months [range 1.9–34.4]): median age 69 yrs; 56% male; 67% primary MF; 63% high-risk MF; 67% JAK2V617F positive; 41% platelet count <100 × 109/L; baseline median spleen volume 3190 mL [1072–7815]; baseline median TSS 19.6 [4.9–46.0]. Eighteen patients were considered resistant by the treating physician (8 lack of response, 3 disease progression, 7 loss of response; median RUX exposure 11.1 months [range 1.9–34.4]) and 9 intolerant (6 hematologic, 3 non-hematologic toxicity; median RUX exposure 9.2 months [2.9–33.2]) to RUX. Median fedratinib exposure to cut-off date: 4 cycles (range 1–10); 19 patients remain on treatment. Spleen RRs were 40% (8/20 PP patients) and 43% (3/7) in patients with baseline platelet count <100 ×109/L. Overall, 19% (5/26 evaluable patients) had a symptom response by Wk 12 (Table). The majority of patients had a reduction in TSS at Wk 12 (Figure).
Summary of efficacy results
| . | RUX resistant(n=18) . | RUX intolerant(n=9) . | Overall(n=27) . |
|---|---|---|---|
| Spleen response [PP] at Wk 12a, n/N (%) | 6/14 (43) | 2/6 (33) | 8/20 (40) |
| Symptom response to Wk 12b, n/N (%) | 3/18 (17) | 2/8 (25) | 5/26 (19) |
| . | RUX resistant(n=18) . | RUX intolerant(n=9) . | Overall(n=27) . |
|---|---|---|---|
| Spleen response [PP] at Wk 12a, n/N (%) | 6/14 (43) | 2/6 (33) | 8/20 (40) |
| Symptom response to Wk 12b, n/N (%) | 3/18 (17) | 2/8 (25) | 5/26 (19) |
patients not evaluable: no post-baseline MRI/CT scan (n=5); no baseline MRI/CT scan (n=1); MRI/CT scan outside time window for Wk 12 assessment (n=1).
1 patient not evaluable: no baseline and at least one post-baseline assessment of TSS.
The most common non-hematologic treatment-emergent adverse events (AEs) were gastrointestinal (nausea 67%; diarrhea 56%; vomiting 48%). Grade 3/4 diarrhea occurred in 2 patients; no Grade 3/4 vomiting or nausea. Anemia was the most common hematologic AE (all grades, 93%; Grade 3, 41%; Grade 4, 0%). Thrombocytopenia (all grades) occurred in 19 (70%) patients; Grade 3 in 6 patients and Grade 4 in 2 patients. One patient had Grade 3 AST elevations and 1 patient had Grade 3 hyperbilirubinemia. Seven patients (26%) discontinued treatment due to AEs. There were 2 deaths on study, both due to disease progression.
Interim results from the JAKARTA-2 study indicate that once-daily fedratinib provides clinical benefit, through reduced splenomegaly and symptom burden, in MF patients previously treated with RUX. The safety profile was acceptable and manageable, with safety results to date consistent with those reported in previous trials of fedratinib. Sponsored by Sanofi.
Harrison:Novartis: Research Funding; Novartis, Sanofi, YM Bioscience, Celgene, SBio, Gilead: Honoraria; Novartis, Sanofi, Shire: Speakers Bureau; Novartis, Sanofi, YM Bioscience, SBio, Gilead: Membership on an entity’s Board of Directors or advisory committees. Jourdan:Sanofi: Honoraria. Kiladjian:Novartis, Celgene, AOP Orphan: Research Funding; Novartis, Sanofi, AOP Orphan: Honoraria; Novartis, Sanofi, AOP Orphan: Membership on an entity’s Board of Directors or advisory committees. Cervantes:Novartis: Speakers Bureau; Novartis and Sanofi: Membership on an entity’s Board of Directors or advisory committees. Niederwieser:Novartis Pharma: Consultancy. Cortes:Incyte, Sanofi: Consultancy; Incyte, Sanofi: Research Funding. Passamonti:Novartis, Celgene, Incyte, Sanofi, Roche: Honoraria. Reiter:Sanofi: Honoraria. Heidel:Novartis Inc.: Honoraria; Novartis Inc.: Research Funding; Novartis Inc.: Membership on an entity’s Board of Directors or advisory committees. Silver:Sanofi: Consultancy; Sanofi: Research Funding; Sanofi: Honoraria. Winton:Sanofi: Research Funding. Gupta:Incyte, Novartis: Consultancy; Incyte, Novartis: Research Funding; Novartis: Honoraria. Gisslinger:AOP Orphan Pharmaceuticals, Novartis, Sanofi, Shire, Celgene, Janssen: Membership on an entity’s Board of Directors or advisory committees; AOP Orphan Pharmaceuticals, Novartis, Sanofi, Shire, Celgene, Janssen: Honoraria. Vannucchi:Novartis: Membership on an entity’s Board of Directors or advisory committees. Talpaz:Novartis, Bristol-Myers Squibb, Ariad, Deciphera: Research Funding; Novartis, Bristol-Myers Squibb, Ariad, Deciphera: Speakers Bureau. Zhang:Sanofi: Employment. Shi:Sanofi: Employment. Mesa:Incyte, Genentech, Lilly, MS Pharma, Gilead: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract