Abstract
Management of acute, painful vaso-occlusive crisis (VOC), the hallmark of sickle cell disease (SCD), continues to be limited to symptomatic relief with oral and/or intravenous (IV) opioids. The efficacy of GMI 1070, a novel pan-selectin inhibitor, was recently evaluated in SCD patients hospitalized for VOC. We report in-depth analysis of pain intensity variation, duration and extent of pain management and other pain-related responses in this study.
A multi-center, randomized, double-blind, placebo-controlled Phase 2 trial of multiple IV doses of GMI 1070 in subjects (12-60 yrs) hospitalized for VOC was conducted. A loading dose to achieve steady state drug level was followed by q12 hours (h) maintenance doses. Study drug dose was doubled per protocol after interim PK analysis. Pain intensity was assessed by a 10 cm Visual Analog Scale (VAS); sustained reduction of 1.5 cm or higher and transition to oral analgesics were included in the composite primary endpoint “time to resolution of VOC,” as were readiness for discharge and time to discharge. Opioid utilization was recorded, converted to Morphine Equivalent Units (MEU) based on standard guidelines, and normalized by body weight. Patient controlled analgesia (PCA), oral analgesic and other therapies were not mandated and reflected institutional and individual practices. Comparisons were made between pooled GMI 1070 (low and high dose) and placebo. Analysis of covariance (ANCOVA), adjusting for sex and age, was used to determine differences between groups. Kaplan-Meier (KM) analysis (log rank test) was performed to compare time to event between treatment groups.
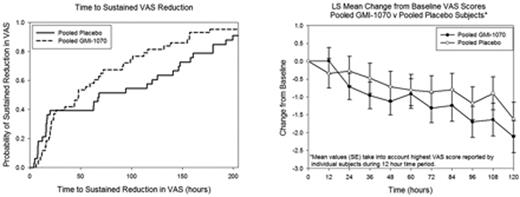
Mean (SD; range) VAS at presentation to ED for all subjects was 8.6 (1.6; 5-10) and decreased before study drug start (baseline) to 6.7 (2.3; 0.8-10), reflecting initial pain management. Mean VAS scores at presentation and at baseline were not different between GMI 1070 and placebo groups. However, VAS changes from baseline by elapsed hours since drug start and probability of sustained 1.5 cm reduction in VAS over time showed that patients receiving GMI 1070 had earlier changes than those in the placebo group, starting at 48 h, (p=0.43, Fig 1a). Similarly, pain intensity reduction was greater by ∼1.5 cm and more rapid within the first 100 h of study treatment for those on GMI 1070 (p=0.58, Fig 1b). In subjects still hospitalized 48 h after study drug initiation, the GMI 1070 group with concomitant hydroxyurea (HU) had the lowest VAS scores. Mean (SD; range) VAS at discharge was 4.03 (2.97; 0-10) in the placebo group and 3.02 (2.75; 0-9.8) in the GMI 1070 group. ANCOVA analysis showed that time to resolution of VOC and time to discharge were shorter in the GMI 1070 group compared to placebo, independent of HU use. Not only was mean [SE] time to transition from parenteral to oral pain medications reduced in the GMI 1070 group vs placebo (108.6 [20.8] vs 155.6 [23.8] h; p=0.14), but active drug exposure was associated with a significant reduction in initial (24 h) and cumulative (during hospitalization) opioid use (oral, IV or both routes) as compared to placebo (Table). Opioid use decreased compared to baseline in the GMI 1070 group within 4 h, while the placebo group did not go below baseline rate until 96h. Duration of IV or oral non-steroidal anti-inflammatory drug (NSAID) use was also lower in the GMI 1070 group vs placebo.
| Analgesic Therapy . | Pooled GMI 1070 Mean (SE) . | Placebo Mean (SE) . | P value . | |
|---|---|---|---|---|
| Oral Opioids MEU/kg | Cumulative | 1.73 (2.2) | 8.25 (2.3) | 0.043 |
| IV Opioids MEU/kg | 1st 24 hours | 1.30 (4.2) | 13.19 (4.8) | 0.065 |
| Cumulative | 9.62 (11.6) | 55.6 (13.1) | 0.010 | |
| IV and Oral Opioids MEU/kg | 1st 24 hours | 1.47 (4.2) | 13.69 (4.9) | 0.060 |
| Cumulative | 11.2 (12.2) | 63.8 (13.9) | 0.006 | |
| IV Opioid duration, days | 4.3 (0.86) | 6.4 (1) | 0.111 | |
| IV or oral NSAID duration, days | 3.9 (0.9) | 6.9 (1.1) | 0.038 | |
| Analgesic Therapy . | Pooled GMI 1070 Mean (SE) . | Placebo Mean (SE) . | P value . | |
|---|---|---|---|---|
| Oral Opioids MEU/kg | Cumulative | 1.73 (2.2) | 8.25 (2.3) | 0.043 |
| IV Opioids MEU/kg | 1st 24 hours | 1.30 (4.2) | 13.19 (4.8) | 0.065 |
| Cumulative | 9.62 (11.6) | 55.6 (13.1) | 0.010 | |
| IV and Oral Opioids MEU/kg | 1st 24 hours | 1.47 (4.2) | 13.69 (4.9) | 0.060 |
| Cumulative | 11.2 (12.2) | 63.8 (13.9) | 0.006 | |
| IV Opioid duration, days | 4.3 (0.86) | 6.4 (1) | 0.111 | |
| IV or oral NSAID duration, days | 3.9 (0.9) | 6.9 (1.1) | 0.038 | |
GMI 1070 therapy was associated with a significant reduction in IV opioid requirement and overall pain medication utilization in subjects hospitalized for SCD VOC. There was also an early decrease in pain intensity as measured by VAS, although this did not reach statistical significance. These effects were independent of HU use and suggest that GMI 1070 has a rapid onset of action and should be investigated for initial and early treatment of VOC in SCD, with the goal of reducing pain intensity and duration, as well as need for opioid therapy.
De Castro:Novella Clinical: Consultancy; GlycoMimetics, Inc.: Research Funding. Wun:Emmaus, Inc.: Clinical Adjudication Committee Other; Pfizer, Inc.: Consultancy; GlycoMimetics: Research Funding. Lanzkron:GlycoMimetics, Inc.: Research Funding. Smith:GlycoMimetics, Inc.: Research Funding. Hassell:Glycomimetics, Inc: Research Funding. Kutlar:GlycoMimetics, Inc: Research Funding. Smith-Whitley:GlycoMimetics, Inc: Research Funding. Rhee:GlycoMimetics, Inc.: Research Funding; Rho, Inc.: Employment. Telen:GlycoMimetics, Inc.: Research Funding; Dilaforette, NA: Research Funding; Pfizer, Inc.: Consultancy. Thackray:GlycoMimetics, Inc.: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract