Abstract
Prior studies examining the incidence of venous thromboembolism (VTE) in lymphoma patients have been limited by small sample size and restriction to specific lymphoma subtypes, leading to a wide range of estimates (1.5%-59.5%). Incidence of VTE in lymphoma patients may be influenced by a variety of patient, clinical, and treatment-related factors. These factors also can influence risk of death, a nuance ignored in prior work. We examined the incidence and predictors of VTE among lymphoma patients in Denmark, considering death as a competing risk.
Our nationwide cohort study identified all newly diagnosed lymphoma patients in Denmark from 2000-2010 using a population-based lymphoma registry (n=10,759), which prospectively collects clinical, pathological, treatment, and relapse and progression data. These data were linked to the Danish National Registry of Patients to identify selected comorbid conditions, treatments, incident VTE (excluding patients with a VTE registered before the year preceding lymphoma diagnosis, n=256), and vital status. VTE incidence rates per 1,000 person-years and 95% confidence intervals (CIs) were computed for pre-specified time intervals from 1 year before through 2 years following lymphoma diagnosis. After excluding patients diagnosed with a VTE during the year before lymphoma diagnosis (n=128), we estimated the 2-year cumulative incidence of VTE overall and by lymphoma subtype, accounting for death as a competing risk. Using the Fine and Gray model, we estimated subdistribution hazard ratios (sHRs) and 95% CIs to identify patient, clinical, and time-dependent treatment predictors of VTE.
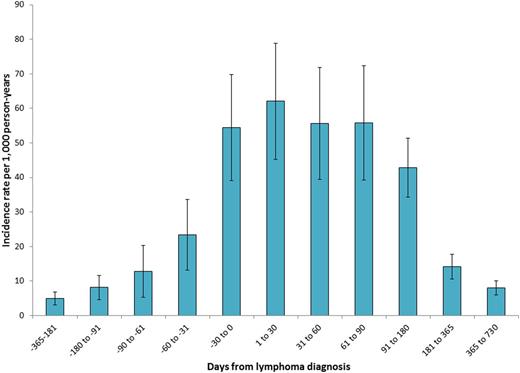
The VTE incidence rate varied over time (Figure 1), with a peak immediately following diagnosis.
Excluding patients with a VTE prior to lymphoma diagnosis, the 2-year cumulative incidence was 3.5% and was highest among peripheral T-cell (5.2%) and diffuse large B-cell (4.5%) lymphoma patients. The overall 2-year cumulative incidence of death was 24.7%.
VTE incidence rates and 95% confidence intervals for one year before and two years after lymphoma diagnosis.
VTE incidence rates and 95% confidence intervals for one year before and two years after lymphoma diagnosis.
presents adjusted sHRs for independent associations between patient, clinical, and treatment characteristics and incident VTE.
| Patient, clinical or treatment characteristic . | Adjusted sHR . | 95% CI . |
|---|---|---|
| Female vs. male | 1.23 | (0.98, 1.55) |
| Age 60+ vs. age<60 years | 1.27 | (0.99, 1.63) |
| Hodgkin vs. DLBCL | 0.90 | (0.62, 1.31) |
| Peripheral T-cell vs. DLBCL | 1.20 | (0.78, 1.85) |
| Non-Hodgkin (not otherwise specified) vs. DLBCL | 0.71 | (0.36, 1.40) |
| Low-risk vs. DLBCL | 0.65 | (0.47, 0.90) |
| Mantle cell vs. DLBCL | 0.52 | (0.28, 0.99) |
| Stage III/IV vs. Stage I/II | 1.09 | (0.83, 1.42) |
| B symptoms (yes vs. no) | 0.82 | (0.62, 1.09) |
| Extranodal involvement (2+ vs. <2 sites) | 0.85 | (0.66, 1.10) |
| CNS (yes vs. no) | 2.22 | (1.32, 3.74) |
| Performance status, 1 vs. 0 | 1.40 | (1.06, 1.85) |
| Performance status, 2 vs. 0 | 0.97 | (0.62, 1.52) |
| Performance status, 3-4 vs. 0 | 1.17 | (0.73, 1.85) |
| LDH above reference level (yes vs. no) | 1.32 | (1.03, 1.69) |
| Platelet count above normal range (yes vs. no)a | 0.93 | (0.65, 1.35) |
| Leucocyte count above normal range (yes vs. no)b | 0.97 | (0.75, 1.27) |
| Myocardial infarction (yes vs. no)c | 1.01 | (0.42, 2.41) |
| Chronic heart failure (yes vs. no)c | 0.86 | (0.41, 1.82) |
| Chronic obstructive pulmonary disease (yes vs. no)c | 1.18 | (0.73, 1.88) |
| Diabetes (yes vs. no)c | 1.21 | (0.75, 1.96) |
| Chemotherapy (time-dependent) | 2.07 | (1.41, 3.06) |
| Central venous catheter (time-dependent) | 1.64 | (1.16, 2.32) |
| Radiation therapy (time-dependent) | 0.84 | (0.59, 1.19) |
| Patient, clinical or treatment characteristic . | Adjusted sHR . | 95% CI . |
|---|---|---|
| Female vs. male | 1.23 | (0.98, 1.55) |
| Age 60+ vs. age<60 years | 1.27 | (0.99, 1.63) |
| Hodgkin vs. DLBCL | 0.90 | (0.62, 1.31) |
| Peripheral T-cell vs. DLBCL | 1.20 | (0.78, 1.85) |
| Non-Hodgkin (not otherwise specified) vs. DLBCL | 0.71 | (0.36, 1.40) |
| Low-risk vs. DLBCL | 0.65 | (0.47, 0.90) |
| Mantle cell vs. DLBCL | 0.52 | (0.28, 0.99) |
| Stage III/IV vs. Stage I/II | 1.09 | (0.83, 1.42) |
| B symptoms (yes vs. no) | 0.82 | (0.62, 1.09) |
| Extranodal involvement (2+ vs. <2 sites) | 0.85 | (0.66, 1.10) |
| CNS (yes vs. no) | 2.22 | (1.32, 3.74) |
| Performance status, 1 vs. 0 | 1.40 | (1.06, 1.85) |
| Performance status, 2 vs. 0 | 0.97 | (0.62, 1.52) |
| Performance status, 3-4 vs. 0 | 1.17 | (0.73, 1.85) |
| LDH above reference level (yes vs. no) | 1.32 | (1.03, 1.69) |
| Platelet count above normal range (yes vs. no)a | 0.93 | (0.65, 1.35) |
| Leucocyte count above normal range (yes vs. no)b | 0.97 | (0.75, 1.27) |
| Myocardial infarction (yes vs. no)c | 1.01 | (0.42, 2.41) |
| Chronic heart failure (yes vs. no)c | 0.86 | (0.41, 1.82) |
| Chronic obstructive pulmonary disease (yes vs. no)c | 1.18 | (0.73, 1.88) |
| Diabetes (yes vs. no)c | 1.21 | (0.75, 1.96) |
| Chemotherapy (time-dependent) | 2.07 | (1.41, 3.06) |
| Central venous catheter (time-dependent) | 1.64 | (1.16, 2.32) |
| Radiation therapy (time-dependent) | 0.84 | (0.59, 1.19) |
sHR=subdistribution hazard ratio, DLBCL=diffuse large B-cell lymphoma, LDH=lactate dehydrogenase
Above normal is >450 mia/L.
Above normal is >10 mia/L.
Assessed during the 5-year period prior to lymphoma diagnosis.
CNS involvement, elevated lactate dehydrogenase count, central venous catheter placement, and chemotherapy were the strongest predictors of VTE, after accounting for risk of death. Patients with low-risk or mantle cell lymphomas were at decreased risk of VTE compared with diffuse large B-cell lymphoma patients. Risks were similar for all other lymphoma subtypes.
Although the incidence of VTE was high among lymphoma patients in Denmark, this risk was substantially lower than the risk of death. In order to mitigate VTE risk while continuing to treat patients with lymphoma, prophylactic approaches should be targeted towards patient subgroups at highest risk.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract