Key Points
The incidence of acute HEV infection after alloHSCT is relatively low, in contrast to a high probability of developing chronic hepatitis.
HEV infection or reactivation should be included in the differential diagnosis of liver enzyme abnormalities in alloHSCT recipients.
Abstract
Hepatitis E virus (HEV) is increasingly acknowledged as a cause of hepatitis in healthy individuals as well as immunocompromised patients. Little is known of HEV infection in recipients of allogeneic hematopoietic stem cell transplantation (alloHSCT). Therefore, we set out to study the incidence and sequelae of HEV as a cause of hepatitis in a recent cohort of 328 alloHSCT recipients. HEV RNA was tested in episodes of liver enzyme abnormalities. In addition, HEV RNA and HEV serology were assessed pre- and post-alloHSCT. We found 8 cases (2.4%) of HEV infection, of which 5 had developed chronic HEV infection. Seroprevalence pre-alloHSCT was 13%. Four patients died with HEV viremia, with signs of ongoing hepatitis, having a median time of infection of 4.1 months. The 4 surviving patients cleared HEV after a median period of 6.3 months. One patient was diagnosed with HEV reactivation after a preceding infection prior to alloHSCT. Although the incidence of developing acute HEV post-alloHSCT is relatively low, the probability of developing chronic hepatitis in severely immunocompromised patients is high. Therefore, alloHSCT recipients should be screened pretransplantation by HEV serology and RNA. Furthermore, a differential diagnosis including hepatitis E is mandatory in all alloHSCT patients with severe liver enzyme abnormalities.
Introduction
In 1983, a new waterborne hepatitis agent was found after an outbreak of unexplained hepatitis at a military camp, later identified as hepatitis E virus (HEV). HEV is endemic in resource-limited countries and an emerging health issue in industrialized countries.1,2 It is a causative agent of acute and chronic hepatitis, transmitted via fecal–oral route, with a mostly self-limiting course in healthy individuals. In human HEV infection, there are 4 known genotypes prevalent, with genotypes 1 and 2 responsible for large waterborne HEV outbreaks in developing countries (Africa and Asia), and genotypes 3 and 4 generally seen in sporadic cases as a zoönotic infection in industrialized countries.1,3 Since the first evidence of chronic hepatitis due to HEV in recipients of solid organ transplants, an increasing awareness for HEV has become apparent.4,5
Persistent chronic infection and cirrhosis have been reported in immunocompromised patients, with most cases in solid organ transplant recipients.5 However, HEV was recently also reported in recipients of allogeneic hematopoietic stem cell transplantation (alloHSCT).6-9 A prevalence of 1% to 3% of hepatitis E viremia in recipients of solid organ transplants has been reported, with 47% to 83% of the patients developing chronic hepatitis.10-13 So far, the incidence and sequelae of hepatitis due to HEV in recipients of alloHSCT is largely unknown.
After 2 recent cases of HEV infection in our clinic, we set out to retrospectively evaluate the point prevalence and clinical sequelae of HEV infection in a cohort of alloHSCT recipients in our clinic, and we studied the role of HEV in transplant recipients presenting with liver enzyme abnormalities.
Methods
Sample collection
We conducted a retrospective cross-sectional analysis of all adult alloHSCT recipients transplanted in the period January 2006 to July 2011, whose serum or EDTA-plasma samples were available in the biobank of Erasmus Medical Center (ErasmusMC; Rotterdam, The Netherlands). These samples, stored at −20°C or −80°C, had been collected during routine visits to our outpatient clinic for clinical assessment of cytomegalovirus (CMV) and Epstein-Barr virus (EBV) reactivation. To select samples, we performed a Laboratory Information Management System database search for last pretransplantation and most recent posttransplantation sample availability. In addition to the cross-sectional analysis, samples were selected from patients experiencing episodes with alanine transaminase (ALT) abnormalities grades 2 to 4, according to Common Terminology Criteria for Adverse Events (version 3.0). Common Terminology Criteria for Adverse Events grades 2 to 4 ALT abnormalities are defined as at least 2.5 times the upper limit of normal. This study was approved by the medical ethical committee (MEC) of ErasmusMC (MEC approval: 2012-522). This study was conducted in accordance with the Declaration of Helsinki.
Virological parameters
For detection of both HEV-specific immunoglobulin M (IgM) and immunoglobulin G (IgG) in serum or plasma samples, the commercially available HEV enzyme-linked immunosorbent assays (Wantai, Beijing, People’s Republic of China) were used. Available peripheral blood, feces, and cerebrospinal fluid (CSF) samples of HEV RNA positive patients were retrospectively analyzed during the course of infection to study the kinetics of serum antibody responses (IgM and IgG) and viremia in different body compartments.
All samples were screened for HEV RNA by an internally controlled quantitative real-time reverse transcription–polymerase chain reaction (RT-PCR), described previously.13 The RT-PCR had a lower limit of detection (95% hit rate) of 143 (2.16 log) IU/mL as determined by the first World Health Organization standard for HEV RNA nucleic acid amplification testing–based assays (6329/10; Paul Ehrlich Institute, Langen, Germany). Phylogenetic analysis was performed to determine genotype, to exclude a common source of infection, and to examine potential HEV reactivation. Statistical analysis and data collection were performed using Microsoft Office Excel 2007 and SPSS (version 20).
Case definition
A case of HEV infection was defined as a patient with an HEV RNA positive serum or plasma sample and was confirmed either by showing HEV-specific serum IgM or IgG antibody response or by showing the presence of HEV RNA in sequential samples. Chronic infection was diagnosed by retrospective testing of stored samples of identified cases and was defined as having HEV viremia of more than 6 months.
Results
Patient characteristics
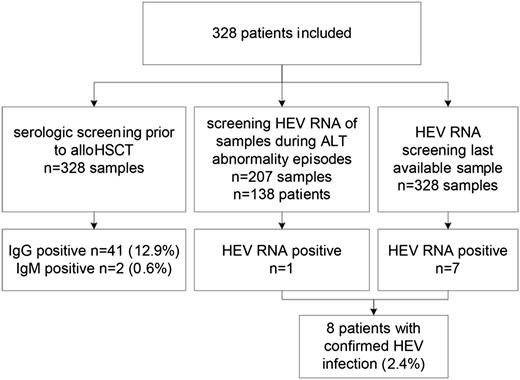
A total of 207 episodes of acute ALT abnormalities, occurring in 138 out of 328 alloHSCT recipients, were evaluated, in addition to a cross-sectional RT-PCR analysis of all 328 patients (Figure 1). As delineated in Table 1, the cohort included 178 (54%) male and 150 (46%) female patients with a median age at transplantation of 50 (range, 17-66) years. Stem cell sources included sibling donors (n=145, 44%), adult matched unrelated donors (MUD) (n = 137, 42%), and umbilical cord blood (UCB) grafts (n = 46, 14%). Acute myeloid leukemia was the most frequent diagnosis for transplantation (n = 142, 43%), followed by acute lymphoblastic leukemia (n = 49, 15%) and non-Hodgkin’s lymphoma (n = 31, 9%). All patients received graft versus host disease (GVHD) prophylaxis with a combination of a calcineurin inhibitor (cyclosporine A) and mycophenolate according to local policy. Acute GVHD grades II to IV occurred in 130 (40%) patients, and chronic extensive GVHD was present in 122 (37%) patients. At the time of analysis (December 2012), 180 (55%) patients were still alive, with a median follow-up of 40.9 (range, 10-77) months from alloHSCT.
Virological parameters
In total, 8 (2.4%) cases of confirmed HEV infection were found in 328 patients, of which 7 (88%) were identified by cross-sectional analysis, and 1 (13%) by screening the episodes of acute ALT abnormalities.
HEV-specific IgG prior to alloHSCT was detected in 41 (13%) patients. Two (0.6%) patients were IgM positive, though HEV viremia could not be confirmed by RT-PCR. Presence or absence of HEV-specific antibodies (both IgM and IgG) prior to alloHSCT was not predictive for HEV infection after alloHSCT, tested by Pearson’s χ2 test of independency (P = .313).
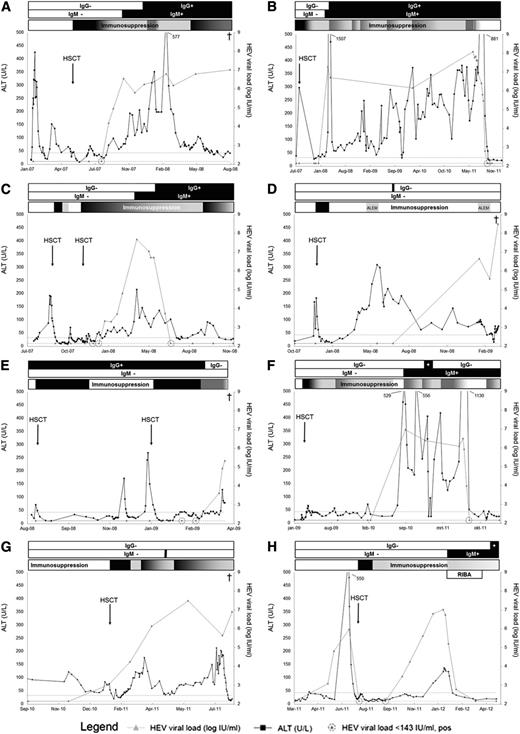
The courses of HEV infection of all 8 cases are presented in Figure 2. Clinical and virological features are delineated in Table 2. Patients will be annotated according to their assigned letter: patients A–H. Within the 8 cases, complete HEV-IgM and -IgG seroconversion occurred in 5 patients, of whom 4 eventually cleared the virus and 1 deceased with an HEV viremia (patients A-C, F, H). Median time from first HEV RNA detection to HEV-IgM and HEV-IgG conversion of these patients was 65 (range, 0-245) days, and 126 (range, −594 to 351) days, respectively. Three patients, who all died with HEV viremia, had aberrant serodynamics: 1 patient did not have detectable HEV-IgG, with only 1 serum sample testing HEV-IgM positive (patient G). Two patients did not have detectable HEV-IgM levels (patients D, E). One of them had detectable HEV-IgG in only 1 sample (patient D), and 1 had detectable HEV-IgG levels at time of alloHSCT, though declining to undetectable at the time of death 7 months later (patient E).
Courses of hepatitis E infection in all 8 individual patients. (A) HEV RNA persisted, although HEV-IgM and HEV-IgG seroconversion occurred under immunosuppressive therapy. This patient died of therapy refractory progressive gastrointestinal GVHD with concurrent chronic HEV infection. (B) Acute ALT abnormalities arose during HEV infection. This patient was mistakenly diagnosed as hepatic GVHD, and immunosuppression was intensified multiple times because of persisting liver enzyme abnormalities. This patient cleared HEV with stopping all immunosuppression, after the diagnosis of HEV infection in this study. (C) This patient developed primary graft failure of a 8/8 human leucocyte antigen (HLA)-matched unrelated donor graft after reduced-intensity conditioning with rabbit antithymocyte globulin, fludarabine, and a single donor fraction of 2 gray total body irradiation. HEV RNA was present after second alloHSCT. This patient cleared infection after HEV-IgM and HEV-IgG seroconversion, supported by reduction of immunosuppressive therapy. (D) This patient developed graft failure of a 7/8 HLA-matched unrelated donor graft after reduced-intensity conditioning with rabbit antithymocyte globulin, fludarabine, and a single fraction of 2 gray total body irradiation. Patient’s disease relapsed 3 months after graft failure. Reinduction therapy was started with alemtuzumab (ALEM), and a second alloHSCT was prepared. However, because of recurrent infections, this patient was not able to complete treatment. Patient died shortly after his second cycle of alemtuzumab because of complications of meningitis and secondary sepsis with E coli. Of note, patient’s CSF samples tested positive for HEV. (E) Secondary graft failure occurred 3 months after the first alloHSCT. The second alloHSCT was complicated by multiple respiratory viral and bacterial infections, which eventually led to respiratory failure and death. (F) This patient was diagnosed as hepatic GVHD, and immunosuppression was introduced in August 2010. Patient cleared HEV after cessation of all immunosuppression, following the diagnosis of HEV infection. (G) HEV RNA was detectable at time of alloHSCT, and viral load increased under immunosuppressive therapy until patient died because of a respiratory viral infection. This patient showed HEV-IgM in 1 sample (black bar) and no HEV-IgG seroconversion. (H) HEV reactivation occurred after initial undetectable HEV RNA without seroconversion. After reduction of immunosuppressive therapy and addition of ribavirin (RIBA), the patient seroconverted and cleared HEV. The ALT upper limit of normal is 33 U/L and 44 U/L for females and males, respectively. The HEV RNA lower limit of detection is 143 (2.16 log) IU/mL.
Courses of hepatitis E infection in all 8 individual patients. (A) HEV RNA persisted, although HEV-IgM and HEV-IgG seroconversion occurred under immunosuppressive therapy. This patient died of therapy refractory progressive gastrointestinal GVHD with concurrent chronic HEV infection. (B) Acute ALT abnormalities arose during HEV infection. This patient was mistakenly diagnosed as hepatic GVHD, and immunosuppression was intensified multiple times because of persisting liver enzyme abnormalities. This patient cleared HEV with stopping all immunosuppression, after the diagnosis of HEV infection in this study. (C) This patient developed primary graft failure of a 8/8 human leucocyte antigen (HLA)-matched unrelated donor graft after reduced-intensity conditioning with rabbit antithymocyte globulin, fludarabine, and a single donor fraction of 2 gray total body irradiation. HEV RNA was present after second alloHSCT. This patient cleared infection after HEV-IgM and HEV-IgG seroconversion, supported by reduction of immunosuppressive therapy. (D) This patient developed graft failure of a 7/8 HLA-matched unrelated donor graft after reduced-intensity conditioning with rabbit antithymocyte globulin, fludarabine, and a single fraction of 2 gray total body irradiation. Patient’s disease relapsed 3 months after graft failure. Reinduction therapy was started with alemtuzumab (ALEM), and a second alloHSCT was prepared. However, because of recurrent infections, this patient was not able to complete treatment. Patient died shortly after his second cycle of alemtuzumab because of complications of meningitis and secondary sepsis with E coli. Of note, patient’s CSF samples tested positive for HEV. (E) Secondary graft failure occurred 3 months after the first alloHSCT. The second alloHSCT was complicated by multiple respiratory viral and bacterial infections, which eventually led to respiratory failure and death. (F) This patient was diagnosed as hepatic GVHD, and immunosuppression was introduced in August 2010. Patient cleared HEV after cessation of all immunosuppression, following the diagnosis of HEV infection. (G) HEV RNA was detectable at time of alloHSCT, and viral load increased under immunosuppressive therapy until patient died because of a respiratory viral infection. This patient showed HEV-IgM in 1 sample (black bar) and no HEV-IgG seroconversion. (H) HEV reactivation occurred after initial undetectable HEV RNA without seroconversion. After reduction of immunosuppressive therapy and addition of ribavirin (RIBA), the patient seroconverted and cleared HEV. The ALT upper limit of normal is 33 U/L and 44 U/L for females and males, respectively. The HEV RNA lower limit of detection is 143 (2.16 log) IU/mL.
HEV-open reading frame 1b (ORF1b) sequences were generated for all 8 cases and deposited in Genbank under the accession numbers JQ015439, JQ015407, KC171439-KC1714444, KC171447, KC171450, and KC171451. Phylogenetic analysis did not identify a common or nosocomial source of HEV transmission. All HEV isolates were classified within genotype 3, as shown in the phylogenetic tree (Figure 3). Interestingly, confirmed HEV reactivation occurred in 1 patient, as described below (patient H).
Phylogenetic tree of ORF1b HEV sequences in 8 HEV-infected alloHSCT recipients. Phylogenetic relation of 321 bp ORF1b region was calculated using maximum likelihood, Kimura's 2 parameter analysis with bootstrapping (n = 1000). Branch lengths are proportional to the evolutionary relationship between the sequences, and internodal confidence of >70% is depicted in the tree. Genbank accession numbers, country of origin (eg, The Netherlands [NL], United States of America [USA]), HEV study number (eg, HEV001), date of drawal (yyyymmdd), and alloHSCT recipients are indicated in the taxa (red text). No indication for a common origin or for nosocomial HEV transmission was found.
Phylogenetic tree of ORF1b HEV sequences in 8 HEV-infected alloHSCT recipients. Phylogenetic relation of 321 bp ORF1b region was calculated using maximum likelihood, Kimura's 2 parameter analysis with bootstrapping (n = 1000). Branch lengths are proportional to the evolutionary relationship between the sequences, and internodal confidence of >70% is depicted in the tree. Genbank accession numbers, country of origin (eg, The Netherlands [NL], United States of America [USA]), HEV study number (eg, HEV001), date of drawal (yyyymmdd), and alloHSCT recipients are indicated in the taxa (red text). No indication for a common origin or for nosocomial HEV transmission was found.
Characteristics of HEV RNA positive patients
The median age of 8 HEV infected patients was 56 (range, 39-66) years at transplantation, including 5 (63%) males and 3 (37%) females (Table 2). All patients were screened for hepatitis B virus, hepatitis C virus, EBV, adenovirus, varicella zoster virus, herpes simplex virus type 1 and 2, and CMV by PCR to exclude the role of other potential hepatrophic viruses. All tested samples were undetectable by PCR, except for 1 patient experiencing CMV reactivation at the time of HEV infection (patient E). In this patient, HEV viremia persisted after successful treatment with ganciclovir, excluding the role of CMV in hepatitis in this patient. All 8 patients received a graft from an alternative donor, including peripheral blood grafts from an adult MUD in 5 (63%) patients and UCB grafts in 3 (37%) patients. Plasma of the adult MUD grafts was HEV RNA negative. No samples of the UCB grafts were available for HEV RNA screening, yet 2 of 3 UCB recipients were HEV viremic at the time of alloHSCT (patients G, H). Six patients received multiple blood transfusions within 3 months prior to HEV infection, including platelet and red blood cell transfusions. None of the blood products were available for testing for HEV serology or RNA at the time of submission.
The median time from alloHSCT to infection was 4.6 (range, −2 to 18) months. The median peak ALT during HEV infection was 289 (range, 138-1507) U/L. At the time of infection, 6 (75%) patients were receiving intensive immunosuppressive therapy (≥2 agents), prescribed for GVHD prevention (n = 2, 33%) or GVHD treatment (n = 4, 66%). In the HEV-infected patients, liver enzyme abnormalities were thought to be related to hepatic GVHD in 5 (63%) patients, and drug-induced liver injury in 3 (38%) patients.
Four (50%) patients died with persistent HEV viremia and signs of ongoing hepatitis (patients A, D, E, G). Median duration of HEV infection in deceased patients was 4.1 (range, 2-13) months, with acute HEV infection in 3 patients and chronic HEV infection in 1 patient. The cause of death was respiratory failure due to infection (fungal, bacterial, and viral) in 3 patients (patients D, E, G), and 1 patient died of therapy refractory progressive gastrointestinal GVHD (patient A). Of note, one of the deceased patients appeared to have HEV RNA positive CSF with retrospective testing of CSF samples (patient D). These samples were obtained during an episode of meningitis and secondary sepsis with positive CSF and blood cultures for Escherichia coli. Radiological evaluation (computed tomography scan) revealed cerebral ischemia due to infection. This patient eventually died of respiratory failure due to fluid aspiration with a low level of consciousness since the meningitis.
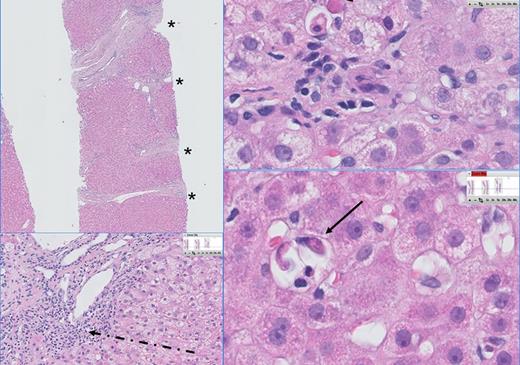
The 4 (50%) living patients cleared HEV infection within a median period of 6.3 (range, 2-42) months (patients B, C, F, H). One patient received ribavirin treatment twice daily with 400 mg for 3 months after a starting dose of 3 times 600 mg daily for 10 days because of a concurrent respiratory syncytial virus infection (patient H). Three patients cleared HEV during cessation of immunosuppressive therapy (patients B, F, H). The cessation rate depended on the presence or occurrence of GVHD. Among living patients, chronic HEV occurred in 3 patients (patients B, C, F), whereas 1 patient was able to clear HEV infection within 6 months (patient H). After HEV diagnosis was confirmed, a liver biopsy was taken from 2 patients (patients B, F), showing hepatitis, severe fibrosis, and portal inflammation (Figure 4). Liver histology was available in 1 patient by autopsy, showing no abnormalities (patient G).
Liver histology of a patient with chronic HEV. The histopathology of chronic HEV infection in this patient is characterized by a dense lymphoplasmocellular infiltrate (dashed arrow) in the portal tracts, combined with severe fibrosis (F3) and portoportal septation (*). Multiple foci of apoptotic bodies are seen in the lobuli surrounded by a few inflammatory cells, indicating individual hepatocyte necrosis (councilman bodies: arrow) and probably caused by viral replication.
Liver histology of a patient with chronic HEV. The histopathology of chronic HEV infection in this patient is characterized by a dense lymphoplasmocellular infiltrate (dashed arrow) in the portal tracts, combined with severe fibrosis (F3) and portoportal septation (*). Multiple foci of apoptotic bodies are seen in the lobuli surrounded by a few inflammatory cells, indicating individual hepatocyte necrosis (councilman bodies: arrow) and probably caused by viral replication.
Remarkably, 1 patient initially cleared the virus and showed reactivation after a period of 53 days of undetectable HEV RNA (patient H). At the time of alloHSCT, HEV RNA was detectable, though viral load was low (<143 IU/mL). The second viremic period was characterized as viral reactivation after alloHSCT, based on identical HEV-ORF1b sequences (Figure 3). This patient finally cleared the reactivated HEV infection within 2 months after diagnosis, supported by ribavirin treatment (as described above) and reduction of immunosuppressive therapy.
Discussion
Recipients of allogeneic stem cell grafts, and especially those receiving alternative donor grafts, are at increased risk of opportunistic bacterial, fungal, and viral infections. Here we describe the first retrospective cross-sectional study of HEV infection in a large cohort of alloHSCT patients. We report a relatively low incidence of 2.4%, in comparison with other opportunistic infections in alloHSCT recipients. Nevertheless, we found a high probability of 63% of developing chronic HEV infection.
Previously, 2 cohorts of 72 and 52 alloHSCT patients were screened for HEV by Abravanel et al6 and Koenecke et al,8 respectively, without positive cases for HEV infection or reactivation, concluding that alloHSCT patients are at low risk for HEV infection and reactivation. However, these 2 cohorts of alloHSCT recipients comprised a more limited number of patients. In our study we identified 8 HEV cases in a larger cohort (n = 328), confirming the HEV prevalence of 2.4% in immunocompromised patients.4,5,11,13 Second, the study of Abravanel et al6 included a restricted follow-up period of 6 months after alloHSCT, whereas our study had a median follow-up time of 41 months. Additionally, misdiagnosing HEV as drug-induced liver injury has been reported previously by Dalton et al,14 whereas this patient group was excluded in the study of Koenecke et al.8 To reduce the risk of missing HEV infections, we screened all patients for HEV RNA at episodes of liver enzyme abnormalities in addition to last available samples. Of the confirmed HEV cases, 5 were misdiagnosed as GVHD, and 3 cases were mistakenly diagnosed as drug-induced liver injury. Diagnosis of HEV in these patients is hampered by relatively low peak aminotransferase levels in comparison with nonimmunocompromised patients,15 which may be explained by intensive immunosuppressive therapy suppressing inflammation.
In our cohort, chronic hepatitis occurred in 5 out of 8 acute HEV cases. However, only 6 patients had sufficient follow-up for a potential diagnosis of chronic hepatitis, because 2 patients died within 2 months after acquiring HEV infection. Progression to chronic HEV in alloHSCT patients may be explained by an impaired immune reconstitution, including insufficient lymphocyte recovery, which are well-known risk factors for posttransplantation infections.16-18 In particular, impaired reconstitution of CD4+ and CD8+ T-cells predispose for infectious morbidity,19 which is confirmed in studies with CMV and EBV viremia, with patients having low specific CMV and EBV CD4+ and CD8+ T-cell counts predisposing for CMV and EBV reactivation, respectively.20,21
Phylogenetic analysis of patient-derived HEV sequences before and after alloHSCT established HEV reactivation in 1 patient. This is the second case of HEV reactivation after alloHSCT described so far in the literature.7 We could not unequivocally demonstrate a reinfection or reactivation in 3 viremic patients having detectable IgG prior to alloHSCT, because no HEV RNA was detected in available samples prior to alloHSCT. Four other patients were seronegative prior to transplantation, suggesting that transmission had occurred after alloHSCT.
In industrialized countries, HEV genotype 3 predominantly infects pigs, wild boars, and deer but also humans and is recognized as a zoönotic agent. However, the main modes of transmission of genotype 3 and 4 viruses remain to be determined.2,3 The source of HEV infection is unclear, but HEV transmission may be enterically (food borne: porcine livers, shellfish), via blood or blood products, mother to child, and although rare, human to human.1,22 Donors and donated blood are not routinely tested for HEV RNA worldwide, although reports of several cohorts in different countries of healthy blood donors reported HEV RNA and HEV-IgM reactivity, suggesting active infection.23-25 In our cohort, transmission of HEV by blood products cannot be excluded, because 6 out of 8 viremic patients received multiple blood transfusions. Unfortunately, none of these blood products were available for testing at the time of submission.
The high probability of developing chronic HEV found in this study was consistent with other studies in recipients of solid organ transplants.4,5,11,13 HEV-infected patients are at risk of progression to fibrosis (67%) in 1 year from infection,11 and also cirrhosis (10%).10 Therefore immunocompromised patients should be screened prior to transplantation, and during episodes of liver enzyme abnormalities, posttransplantation. In our study, patients showed aberrant serology, which may be explained because of their impaired immune reconstitution. Thus, HEV RT-PCR testing is the preferred diagnostic method in these immunocompromised patients.
Treatment of HEV infection after transplantation includes reduction of immunosuppressive therapy, and there is no registered drug therapy. Anecdotal evidence supports the use of oral ribavirin in immunocompromised patients. In our study, 3 patients cleared HEV with a dose reduction of immunosuppressive agents (ie, cyclosporine A or prednisone) alone. Treatment with ribavirin should be considered in patients for whom immunosuppression cannot be reduced, such as, for example, patients with active GVHD. The optimal daily dose of ribavirin is unknown; in case reports sustained viral response has been described with daily dosages between 200 mg and 1200 mg.11,26 If HEV infection is confirmed prior to alloHSCT, it can be considered as a contraindication to transplantation. Clearance of HEV viremia is therefore of high importance. AlloHSCT candidates are usually pretreated with chemotherapy, resulting in impaired or delayed immune reconstitution. Therefore, early ribavirin treatment can be initiated to support rapid HEV clearance in these future alloHSCT recipients.
In conclusion, this study shows that recipients of alloHSCT are at risk for HEV infection, albeit with a relatively low risk. However, the probability of developing severe chronic hepatitis in immunocompromised patients is high. Therefore, patients should be screened for HEV antibodies and HEV RNA prior to alloHSCT, and patients with acute liver enzyme abnormalities after alloHSCT should be analyzed for HEV reactivation and infection. Moreover, HEV should be included in the differential diagnosis of liver GVHD and drug-induced liver injury, because of the largely overlapping picture with respect to liver enzyme abnormalities.
Presented at the 54th Annual Meeting of the American Society of Hematology, Atlanta, GA, December 8–11, 2012.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Jaap Kuipers and Ronnie van der Holt are acknowledged for their assistance in completing clinical data. Mark Pronk, Manon Briede, Mark Verbeek, and Sevgi Deniz are acknowledged for their technical assistance.
This study was supported by the Virgo consortium, funded by the Dutch government (FES0908), the Netherlands Genomics Initiative (NGI) project number 050-060-452, and by the European Community Seventh Framework Programme (FP7/2007-2013) under project EMPERIE (grant agreement no. 223498).
Authorship
Contribution: S.D.P., H.J.A., A.D.M.E.O., J.J.C., and A.A.v.d.E. contributed to the study design; J.V., H.J.A., R.A.d.M., and J.J.C. were involved in collecting clinical data; S.D.P., J.M., and A.A.v.d.E. were involved in performing virological tests; M.E.I.S. was involved in reviewing histopathology; and J.V., S.D.P., J.J.C., and A.A.v.d.E. were involved in analyzing and interpreting the data and writing this report.
Conflict-of-interest disclosure: A.D.M.E.O. is chief science officer of Viroclinics Biosciences BV, a contract research organization that collaborates with pharmaceutical companies. The remaining authors declare no competing financial interests.
Correspondence: A. A. van der Eijk, Department of Virology, Erasmus University Medical Center, Gravendijkwal 230, 3015 CE, Rotterdam, The Netherlands; e-mail: a.vandereijk@erasmusmc.nl.
References
Author notes
J.V. and S.D.P. contributed equally to this study.

![Figure 3. Phylogenetic tree of ORF1b HEV sequences in 8 HEV-infected alloHSCT recipients. Phylogenetic relation of 321 bp ORF1b region was calculated using maximum likelihood, Kimura's 2 parameter analysis with bootstrapping (n = 1000). Branch lengths are proportional to the evolutionary relationship between the sequences, and internodal confidence of >70% is depicted in the tree. Genbank accession numbers, country of origin (eg, The Netherlands [NL], United States of America [USA]), HEV study number (eg, HEV001), date of drawal (yyyymmdd), and alloHSCT recipients are indicated in the taxa (red text). No indication for a common origin or for nosocomial HEV transmission was found.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/6/10.1182_blood-2013-03-492363/4/m_1079f3.jpeg?Expires=1767965934&Signature=JTEj65ySCAPpjkW-naDWtCGdETWQjsMNAAtn3WXCbf1b5sxxKu52i4KuWAJM8wMEXPv8GR2ZzSAgawy1kRblVGkb-7XEtOUQAD9IVgWI319EjnESoniuqQ~TFPVHc7NxzBxA8LQSbqNNjVcgxVQuDfsVXn~4wh5qFaUHoJwSopb~wbIK-qHl3tcz1wEppqsB~GoTueCQeLo5AIKmmM8FV0lSXsM4L04KzXkairxWLE7cPu6JxbaUwtvgku23h8SCEEJ9wU2~iEwsyBj79W3sp7~iCpeqGbQNLdFKWH~orMkHcWPMyTiMNxxyq5VCaFatMSjVZo0IniUokfrAJzu8OA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)