Abstract
Cold agglutinin disease is a rare and poorly understood disorder affecting 15% of patients with autoimmune hemolytic anemia. We reviewed the clinical and pathologic features, prognosis, and management in the literature and describe our institutional experience to improve strategies for accurate diagnosis and treatment. Retrospective analysis identified 89 patients from our institution with cold agglutinin disease from 1970 through 2012. Median age at symptom onset was 65 years (range, 41 to 83 years), whereas the median age at diagnosis was 72 years (range, 43 to 91 years). Median survival of all patients was 10.6 years, and 68 patients (76%) were alive 5 years after the diagnosis. The most common symptom was acrocyanosis (n = 39 [44%]), and many had symptoms triggered by cold (n = 35 [39%]) or other factors (n = 20 [22%]). An underlying hematologic disorder was detected in 69 patients (78%). Thirty-six patients (40%) received transfusions during their disease course, and 82% received drug therapy. Rituximab was associated with the longest response duration (median, 24 months) and the lowest proportion of patients needing further treatment (55%). Our institution’s experience and review of the literature confirms that early diagnostic evaluation and treatment improves outcomes in cold agglutinin disease.
Introduction
Autoimmune hemolytic anemias (AIHAs) consist of warm-, cold-, or mixed-reactive antibody types that are directed against antigens on the red blood cell (RBC) surface.1 The autoantibodies may be idiopathic (primary) or related to an underlying condition such as infection, malignancy, or immune disease (secondary).2 Cold-antibody AIHAs are further characterized into cold agglutinin disease (CAD) and paroxysmal cold hemoglobinuria.3 CAD is rare, accounting for 15% of AIHA cases,4 with an incidence of 1 per million people per year.5 CAD has a low prevalence, and most published reports are small retrospective series, case reports, or expert opinions.5 Since 1960, our institution has seen more than 43 000 patients with monoclonal gammopathies, but less than 1% of these involved cold-reactive autoantibodies.6 Here, we review the clinical and pathologic features, prognosis, and management of CAD described in the literature, and we also review our institutional experience with CAD.
History and mechanism
At the turn of the 20th century, Landsteiner7 first described blood agglutination at cold temperatures. Subsequently, Clough and Richter8 in 1918 identified the pathologic association of cold agglutination with RBC breakdown and its occurrence with respiratory infections. In 1943, Horstmann and Tatlock9 reported detecting cold agglutinins in the serum of patients with primary atypical pneumonia. Fifty years after Landsteiner’s findings, the term “cold agglutinin disease” was coined by Schubothe.10
The monoclonal protein responsible for CAD was identified as having antibody activity by Christenson and Dacie.11,12 These monoclonal proteins could be removed by incubating the serum with RBCs bearing the I antigen. In 1960, Marsh and Jenkins13 described 2 patients whose sera agglutinated adult I-negative (i) cells strongly but agglutinated normal (I) adult cells weakly.
CAD in 90% of patients is an immunoglobulin M (IgM) –mediated process, with rare findings of monoclonal IgG, IgA, or λ light chain restriction, whereas warm AIHA is predominantly an IgG-driven disease.5,14,15 The term “cold agglutinin” arises less from the clinical symptomatology and instead refers to the finding of agglutination without antiglobulin antisera in microtiter wells at 4°C. This occurs because IgM is a 1-million-Da molecule capable of spanning the distance between RBCs and overcoming the natural repulsive forces between cells, thus allowing spontaneous in vitro agglutination. In the body core, circulating IgM remains unbound from the RBC surface. However, as blood shifts toward the peripheral circulation and cools, IgM transiently binds the RBC membrane. Once bound, the IgM molecule activates the complement cascade, binding C3b to the cell surface. As C3b-coated cells return toward the body core, IgM dissociates. C3b-coated cells subsequently lose surface membrane by receptor-specific macrophages present predominantly in the liver (but also to a lesser degree in the spleen), resulting in extravascular hemolysis and perhaps some degree of intravascular hemolysis. The severity of hemolysis depends on the thermal amplitude, rather than the serum concentration of IgM.16-18
Cold agglutinin IgM molecules can be polyclonal or monoclonal, with each associated with a different origin and prognosis. Polyclonal antibodies, typically seen in the postinfectious setting, are self-resolving, although treatment of the underlying infection may hasten resolution. Postinfectious cold agglutinins are seen with viral and bacterial pathogens, including mycoplasma,19 Epstein-Barr virus,20-22 and legionella. Case reports of cold agglutinin hemolysis induced by varicella,23-25 Citrobacter,26 and influenza27,28 have been described. Polyclonal IgM antibodies are most commonly seen in children. Monoclonal IgM CAD is classically seen in older adults; it is a long-term disease that often resists treatment and may be associated with an underlying lymphoproliferative disorder. Some evidence suggests clonal light chain dominance in 90% of patients with CAD.5 Cytogenetic studies have noted trisomies 3 and 1229 and the t(8;22) mutation.30
The monoclonal cold agglutinin IgM molecules are directed against the I/i carbohydrate antigens on the RBC surface. These cold agglutinins have heavy chains encoded by the immunoglobulin heavy variable (IGHV) 4-34 gene segment,31 which suggests that certain amino acid sequences may have a critical role in receptor recognition. Rarely, antibodies have been noted to target antigens outside the I/i system such as the Pr antigen.
Diagnosis, therapy, and prognosis
CAD usually presents in the seventh decade of life and predominantly affects women.3,5,32,33 Berentsen et al5 studied 86 patients and reported a median age at symptom onset of 67 years (range, 30 to 92 years) and a male:female ratio of 0.55. This is similar to smaller studies that reported median ages of 66 and 72 years.34,35 The survival is similar to an age-matched population (median, 12.5 years after diagnosis).5
Cold agglutinin–mediated hemolysis has been considered a chronic anemia of mild to moderate severity,3,10,23,36,37 but this has been challenged in the largest case series published to date,5,6 with a lower tertile of hemoglobin levels of 8 g/dL and ranging as low as 4.5 g/dL. More than 50% of patients required transfusions, and therapy was considered necessary in 70%.5,6 Fatal complications have been reported, with exacerbations of hemolysis triggered by febrile illness, trauma, or surgery.38-45
In addition to hemolysis, clinical manifestations include cold-induced circulatory symptoms; livedo reticularis, Raynaud disease, acrocyanosis and, rarely, cutaneous necrosis.20,46-49 Berentsen et al5 reported cold-induced symptoms in more than 90% of patients. Splenomegaly is not prominent in CAD.11,23,37 Hemoglobinuria tends to be less severe than that seen in paroxysmal cold hemoglobinuria or warm AIHA.3
The diagnosis of CAD is established with hemolytic anemia, reticulocytosis, hyperbilirubinemia, elevated lactate dehydrogenase, and positive Coombs testing for anti-C3 and classically negative anti-IgG. Chandesris et al50 studied 58 patients and reported positivity for anti-C3d antibodies (74%), anti-C3d antibodies + IgG (22%), and IgG alone (3%). After test findings suggest CAD, the antibody titer and thermal activity should be determined.3 The latter is essential to prevent overdiagnosis, because most agglutinins are clinically insignificant; one study showed only 14 (8%) of 172 patients with cold agglutinins displaying clinically significant activity.51 The titer level is less concordant with disease activity because hemolysis occurs with levels as low as <1:325 ; however, most consider titer levels greater than 1:512 as clinically significant.2,52 Berentsen et al5 reported a median titer of 1:2048, whereas Stone and colleagues51 observed titers from 1:512 to 1:65 536. After CAD is established, patients should be evaluated for infections, underlying malignancies, and autoimmune disease because more than 70% of cases may be attributable to these processes.2,6,53 CAD has been associated with lupus54,55 and rheumatoid arthritis.56
The IgM antibodies resulting in classical CAD are monoclonal, and the differential diagnosis includes disorders such as IgM monoclonal gammopathy of undetermined significance (MGUS) and Waldenström macroglobulinemia.38 Immunohistochemical and morphologic evaluation has shown 50 of 66 patients with a non-Hodgkin B-cell lymphoma, with half being lymphoplasmacytic lymphoma.5 Go et al34 observed a lymphoproliferative disorder in 8 of 18 patients with CAD. CAD has also been described in patients with sarcomas,57 carcinomas,58 and melanoma,59 although these cases may be coincidental rather than causal associations. Bone marrow examination for the exclusion of an associated lymphoproliferative disorder is required.
Nonpharmacologic measures are the cornerstone of management of CAD. Recommendations include avoiding cold exposure, increasing use of warm clothing, and possibly relocating to warm regions.10,23,37,60 However, no trials have evaluated the efficacy of these strategies. The thermal amplitude of the cold agglutinin antibody in many patients is such that physiologic peripheral cooling results in antibody binding sufficient to cause anemia.52 Supportive transfusions may be used in patients with severe anemia. An in-line blood warmer should be considered to minimize cold agglutinin binding to transfused red cells.1,52 Cold agglutinin antibodies complicate crossmatch because agglutination causes difficulty in detecting blood type and alloantibodies.52
Previous therapies aimed to suppress production of aberrant IgM protein. Treatments included corticosteroids, alkylating agents, and purine nucleoside analogs. Case series of corticosteroid therapy in CAD report response rates no greater than 14%.5,23,35,61,62 The need for high doses of corticosteroids limits their viability as a long-term treatment.62 Several small series have documented a poor clinical response to alkylating agents and have also noted the need for long-term exposure, with clinically significant adverse effects in those who respond.5,35,61 Cladribine has been used and is not efficacious.61,63 Although appropriate in refractory warm AIHA, splenectomy should not be used to treat CAD because hemolysis occurs outside the spleen.23
Rituximab has been used to treat CAD.64-66 Trials of rituximab at a weekly dose of 375 mg/m2 for 4 weeks report response rates of 45% to 58%, with only rare complete responses.5,67-69 Sustained remission is unlikely, and 57% to 89% of responding patients eventually relapse.68,69 One prospective trial noted a median response duration of 11 months (range, 2 to 42 months).68 Rituximab has been used successfully in relapsed CAD.1
Therapy with fludarabine and rituximab has been evaluated in CAD.1 A trial of 29 patients, 10 of whom were unresponsive to rituximab monotherapy, showed a response rate of 76%. Among those refractory to rituximab monotherapy, the response rate was 70%. Among responders, 27% had a complete response and 72% had a partial response. Five patients (23% of responders) relapsed (estimated median response duration, >66 months).70
Eculizumab is a monoclonal antibody used to treat paroxysmal nocturnal hemoglobinuria; it binds to complement protein C5, inhibiting formation of the terminal complement complex. Two case reports described eculizumab use in patients with transfusion-dependent CAD that was refractory to rituximab.71,72 Both patients responded to eculizumab alone and achieved transfusion independence. One report described improvement in CAD after treatment with bortezomib.73
Several series have reviewed the operative management of CAD, given the association between hypothermic circulatory arrest and hemolysis during rewarming.45,74 Patients with a low-titer antibody with low thermal amplitude may need no change in management. Patients with a high titer or high thermal amplitude may need a specialized approach to surgery. The primary management strategies are perioperative plasma exchange with fresh frozen plasma75-77 or IgG,78 warmed products (including bypass priming fluids, blood products, and anesthesia gases), and body-warming blankets.45,76 Intraoperative monitoring has been used to keep the core body temperature above the reactive thermal amplitude of the cold agglutinin antibody to prevent hemolysis.45,74,76,78
Review of 89 patients from a single institution
Many treatment strategies for CAD are not evidence based.2 We review our experience with CAD in the largest case series of CAD published to date.
Methods
The study was approved by the Mayo Clinic Institutional Review Board. All patients gave written, informed consent in accordance with the Declaration of Helsinki, allowing the use of their medical records for research at the time of their initial clinical encounter. Analyses were conducted by P.L.S., and all authors had access to primary data. We retrospectively reviewed all patients with a diagnosis of CAD seen at Mayo Clinic (Rochester, Minnesota) from January 1, 1970, through May 25, 2012.
CAD was defined as chronic hemolysis, positive cold agglutinin titers, and characteristic findings of a direct antiglobulin test. In patients who did not have direct antiglobulin test results available, diagnosis was verified by cold agglutinin titers and a clinical picture consistent with CAD. For patients without cold agglutinin titer data, diagnosis was verified by a positive direct antiglobulin test and clinical picture. Patients with infection as the underlying cause were excluded.
Response to treatment was defined as improvement in clinical symptoms. Responses were assessed according to time to response, duration of response, and decreased transfusion requirements or transfusion independence. Time to response was defined as the time from the start of the therapy cycle to the achievement of any degree of response. Response duration was the time from achievement of response to the start of an alternative therapy or censoring of data.
Adverse effects and adverse event incidence were recorded. Tolerance was defined as no serious adverse effects or adverse events requiring discontinuation of therapy, including infection and hematologic toxicity.
We use the term “purine analogs” to describe fludarabine and cladribine. The term “alkylating agents” includes chlorambucil, cyclophosphamide, melphalan, cyclophosphamide-vincristine-prednisone, and cyclophosphamide-hydroxydaunorubicin-vincristine-prednisone.
Results
Patient characteristics and survival
We identified 89 patients. Table 1 shows baseline patient characteristics and initial laboratory test results. Thirteen patients had positive monospecific anti-C3d antibodies and positive anti-IgG antibodies on Coombs testing. One patient had only positive monospecific anti-IgG antibodies and positive cold agglutinin titers. These patients were compared with the rest of the cohort. No significant differences were identified in baseline characteristics, clinical symptoms, underlying hematologic diagnosis, or survival. Patients with both anti-IgG antibodies and anti-C3d antibodies had a decreased incidence of receiving treatment (57% vs 90%). However, because the therapy administered was similar, these patients were combined for analysis and reporting.
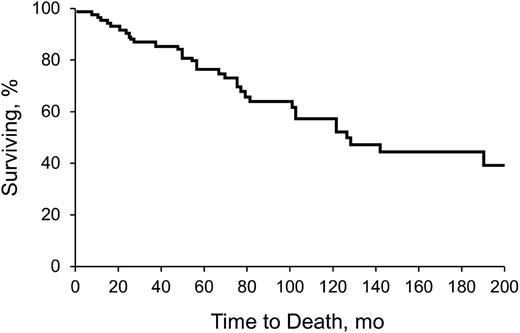
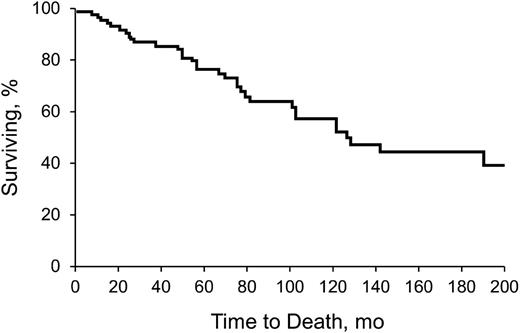
Table 2 shows clinical features, including time to diagnosis, symptoms, triggers, and survival. Table 3 describes underlying hematologic diagnoses. Sixty-nine patients (78%) had a concomitant hematologic disorder associated with CAD. Seven patients (8%) had a third hematologic disease. Development of a third hematologic disorder was mostly in the setting of MGUS (n = 6), and some patients had an unspecified lymphoproliferative disorder (n = 3). Other observed events included macroglobulinemia (n = 2) and unspecified lymphomas (n = 2). The median time to development of a third hematologic disease was 33.9 months (range, 1.5 to 95.4 months). Figure 1 shows the Kaplan-Meier survival curve, from diagnosis.
Kaplan-Meier analysis of survival of all patients with CAD. Analysis began with date of diagnosis.
Kaplan-Meier analysis of survival of all patients with CAD. Analysis began with date of diagnosis.
Therapy
Seventy-three patients (82%) required treatment of CAD. The median time from diagnosis to treatment was 11.4 months (range, 0 to 375.0 months). The most common reason for treatment initiation was progressive anemia (39 patients [53%]), treatment of other CAD symptoms (18 patients [25%]), and treatment of underlying disorder (9 patients [12%]). Twenty-three patients received 1 round of therapy, 19 patients received 2 rounds of therapy, 17 patients received 3 rounds of therapy, 7 patients received 4 rounds of therapy, and 7 patients received 5 or more rounds of therapy. A round of therapy was defined as the continuous use of a single treatment regimen (one or multiple agents), and the duration was measured from the initiation of the treatment regimen through cessation. Transfusions were required for 36 (40%) of 89 patients during the disease course. Table 4 details the therapy and outcomes, including transfusion requirements, response, and need for further therapy.
Five patients underwent splenectomy. This was first-line therapy for only 1 patient. Preoperatively, 1 patient required intermittent blood transfusions. Response data were available for all patients; the response rate was 40%, with a median response duration of 9.9 months (range, 5 to 14.8 months). Four patients (80%) needed further therapy. No change in the need for blood transfusions was noted.
Adverse events
Adverse effects of corticosteroids included severe diabetes mellitus necessitating hospitalization (n = 1) and psychosis (n = 1). Both patients were receiving single-agent prednisone therapy, and medication cessation was necessary.
For rituximab therapy, adverse events were rare. However, anaphylaxis occurred in 2 patients. One was receiving both rituximab and prednisone, and the other was receiving single-agent rituximab. For patients receiving alkylating agents, clinically significant adverse events included neutropenia (n = 3), with 1 patient hospitalized for neutropenic fever.
Discussion
Because CAD is rare, large studies are difficult to conduct. We characterized our clinic’s experience with the disease and explored the efficacy of various therapies. We confirmed the previously reported5,32 disease preponderance in females. Although 94% of CAD patients in our cohort were characterized as white, this may represent referral bias to Rochester, Minnesota. CAD predominantly affected the elderly (median age at diagnosis, 71.6 years) and was associated with a relatively long survival after diagnosis (median, 10.6 years). Although CAD appears indolent when considering the prognosis and degree of lymphoproliferation, the opposite may also be true when considering the associated clinical symptoms and the need for frequent treatment. The insidious nature of CAD was supported by the long duration between symptom onset and diagnosis (median, 37.4 months). The most common presentation was anemia of undetermined origin (median hemoglobin at diagnosis, 10.2 g/L). Although CAD is often characterized by cold-induced symptoms, only 35 patients (39%) in our study described them. This differed from another report that confirmed this characteristic in greater than 90% of patients.5 Possibly, some of our findings were influenced by recall bias or lack of symptom documentation.
Coombs testing is normally positive for polyspecific antibodies and/or monospecific anti-C3d antibodies in CAD. These tests were positive in more than 90% of patients in this study. However, 30% of patients tested had both positive monospecific anti-C3d antibodies and positive monospecific anti-IgG Coombs test results. These patients may reflect a group with mixed warm-cold AIHA or CAD due to atypical autoantibodies. Although these patients appeared to have milder disease and did not require treatment as often as the rest of the cohort, treatment characteristics were similar to those with classic CAD, and thus the groups were combined for analysis and reporting. Past studies have similarly shown IgG on erythrocytes in 21% of patients with CAD.5
In our series, underlying hematologic diseases were identified in 69 patients (76%); of these, MGUS was most common (n = 42 [47%]). The other hematologic disorders were lymphoproliferative disorders (Table 3). MGUS is relatively common (affecting 3% of patients older than age 50 years) and is associated with a broad range of conditions, including macroglobulinemia, plasma cell proliferative disorders, and CAD.52,79,80 This association between CAD and lymphoproliferative disorders corresponds with previous observations5,81 ; like MGUS, this likely represents a monoclonal IgM gammopathy produced by lymphoplasmacytic lymphoma. A third hematologic disease developed in 7 patients, 6 of whom had the initial diagnosis of MGUS. This most likely reflects the clinical progression of MGUS to a malignant plasma cell dyscrasia or other lymphoproliferative disorder.
The patients with CAD and MGUS identified in this case series likely represent a population with an IgM-related disorder. The term “IgM-related disorder” originally was defined to better elucidate the spectrum of paraprotein diseases from Waldenström macroglobulinemia to MGUS.82-84 IgM-related disorders are a clinical group distinct from MGUS and from Waldenström macroglobulinemia. This group includes patients with clinical features attributable to a monoclonal IgM protein, without evidence of bone marrow–infiltrating lymphoma. IgM-related disorders include diseases caused by aggregation of monoclonal proteins such as primary amyloidosis, light chain deposition disease, and type I cryoglobulinemia, as well as diseases attributable to the autoantibody activity of the monoclonal immunoglobulins. This latter group includes neuropathy, type II mixed cryoglobulinemia, scleromyxedema, CAD, polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes (POEMS) syndrome.83 Treatment of IgM-related disorders is dependent on the presenting clinical syndromes. Morra et al85 examined MGUS and IgM-related diseases and observed no significant difference in 10-year survival rates (84.7% and 87.7%, respectively). Malignant transformation occurred in 11.7% of patients with MGUS and 9.5% of patients with IgM-related disease. Development of symptomatic Waldenström macroglobulinemia was the most common transformation.
The most appropriate pharmacotherapy for CAD has remained an area of research. In this study, only 35% of patients treated with single-agent prednisone had a response, and most responders needed further therapy. Corticosteroid-containing treatment regimens had a response rate of 40%, but few patients (31%) subsequently were independent of further therapy. Alkylating agent–containing regimens had a 44% response rate, but a similar low proportion of patients achieved independence from further therapy compared with those being given corticosteroid-containing regimens. Other studies report similarly low rates of clinical response.5,35,61 We observed a response rate of 50% with purine analogs, with 40% of treated patients achieving independence from further therapy. Eighty percent of patients receiving purine analog therapy and 80% of patients undergoing alkylating agent therapy had a second concomitant hematologic diagnosis, but the diagnosis may have been unknown when therapy began (ie, diagnosis may not have been established until later in the disease course).
In this study, rituximab monotherapy and combination therapy were analyzed. Response rates were 83% in single-agent therapy and 79% in combination therapy; this was higher than previously reported rates.5,69 In our cohort, independence from further therapy was achieved by 51% of patients receiving single-agent therapy and 45% receiving combination therapy. Although rituximab generally was well tolerated, 2 had anaphylaxis during administration. Past studies have noted few adverse effects to rituximab, although one described a rare association with progressive multifocal leukoencephalopathy and hepatitis B reactivation.1
It is difficult to draw firm conclusions with the small number of patients included and the low overall pretreatment transfusion rate. However, although all treatments were associated with a decrease in transfusion requirements, our observations suggest that therapies containing rituximab appeared to have the greatest effect on decreasing transfusion requirements.
This study defined response as an improvement in clinical symptoms. Improvement of anemia was not considered for several reasons, including varying availability of serial hemoglobin testing during and after treatment, as well as the lack of a consistent hemoglobin monitoring schedule during therapy. Although this is a limiting factor and may account for some of the variability between our results and those of past studies, we believe that for this study, patient-related outcomes yielded the most reliable and clinically useful end points. Some data heterogeneity likely was attributable to differences among physicians over time and the lack of strict definitions for CAD and treatment response criteria. However, we believe these types of errors were minimized by using structured response end points (ie, need for therapy). As shown in Table 4, dual-agent therapy was quite common. Combination therapy may reflect synergistic efficacy relative to the individual agents. Although we did not analyze specific combinations of therapeutic agents, we did account for the frequency of dual-agent therapy, which may be a useful parameter when comparing agents and their treatment profiles.
Conclusion
We reviewed the origin, diagnosis, and treatment of CAD and also presented original research describing CAD and outcomes associated with certain treatment regimens. CAD is an extravascular hemolytic process mediated by an IgM monoclonal protein commonly associated with lymphoproliferative disorders. It is associated with a relatively long median survival after diagnosis. Rituximab is an efficacious, well-tolerated treatment that is associated with high response rates, although complete and sustained remissions are uncommon.
Acknowledgments
This work was supported in part by research funding from Alexion Pharmaceuticals (M.A.G.).
Authorship
Contribution: P.L.S. and L.T.H. participated in literature review, study design, data collection, data analysis and interpretation, and manuscript writing; and M.A.G. participated in study design, data interpretation, and manuscript editing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Morie A. Gertz, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; email: gertz.morie@mayo.edu.