Key Points
Compared with intermediate-dose prophylaxis (3 × 1000 IU/wk), high-dose prophylaxis (3 × 2000 IU/wk) resulted in a 66% higher total cost.
At age 24 years, high-dose prophylaxis resulted in a small reduction in bleeding and hemophilic arthropathy, but equal quality of life.
Abstract
Prophylactic treatment in severe hemophilia is very effective but is limited by cost issues. The implementation of 2 different prophylactic regimens in The Netherlands and Sweden since the 1970s may be considered a natural experiment. We compared the costs and outcomes of Dutch intermediate- and Swedish high-dose prophylactic regimens for patients with severe hemophilia (factor VIII/IX < 1 IU/dL) born between 1970 and 1994, using prospective standardized outcome assessment and retrospective collection of cost data. Seventy-eight Dutch and 50 Swedish patients, median age 24 years (range, 14-37 years), were included. Intermediate-dose prophylaxis used less factor concentrate (median: Netherlands, 2100 IU/kg per year [interquartile range (IQR), 1400-2900 IU/kg per year] vs Sweden, 4000 IU/kg per year [IQR, 3000-4900 IU/kg per year]); (P < .01). Clinical outcome was slightly inferior for the intermediate-dose regimen (P < .01) for 5-year bleeding (median, 1.3 [IQR, 0.8-2.7] vs 0 [IQR, 0.0-2.0] joint bleeds/y) and joint health (Haemophilia Joint Health Score >10 of 144 points in 46% vs 11% of participants), although social participation and quality of life were similar. Annual total costs were 66% higher for high-dose prophylaxis (mean, 180 [95% confidence interval, 163 - 196] × US$1000 for Dutch vs 298 [95% confidence interval, 271-325]) × US$1000 for Swedish patients; (P < .01). At group level, the incremental benefits of high-dose prophylaxis appear limited. At the patient level, prophylaxis should be tailored individually, and many patients may do well receiving lower doses of concentrate without compromising safety.
Introduction
Patients with severe hemophilia have undetectable factor VIII (FVIII) or IX levels, resulting in spontaneous and trauma-related bleeding, especially in the joints. Repeat joint bleeding eventually leads to a crippling arthropathy. Severe hemophilia is rare, with a prevalence of about 40 cases per million inhabitants. Since its introduction in 1958 by Professor Nilsson in Sweden,1 many long-term observational studies2-5 and 2 pediatric randomized controlled trials6,7 have shown that prophylactic replacement therapy in severe hemophilia prevents bleeds and subsequent hemophilic arthropathy. This was confirmed by the latest version of the Cochrane review on prophylaxis.8 However, the increased use of factor concentrates in prophylaxis and the associated costs (from €72 000 [US$76 700] annually for small children9 to €146 000 [US$155 600] for an adult10 receiving high-dose prophylaxis in the 1990s) have been limiting factors of a more widespread introduction of prophylaxis.
The Swedish regimen originally aimed at maintaining minimum trough levels of clotting factor activity by using doses of 25 to 40 IU/kg 3 times a week for hemophilia A.11 In The Netherlands, however, prophylaxis was introduced in 1968,12 using lower doses and tailoring the on the basis of clinical observation to prevent spontaneous joint bleeds. Although treatment has intensified over the years in both countries,3,13 the difference in dosing has remained considerable: Today, a typical adult Dutch patient with hemophilia A uses 3 × 1000 IU FVIII/week, whereas a typical adult Swedish patient uses 3 × 2000 IU or 1500 IU every other day. Both groups reported favorable long-term results, but with increasing pressure on health care budgets and a formal cost review by the Swedish authorities,14 it is important to assess the incremental gains of high-dose prophylaxis.
Assessment of long-term effects requires decades of follow-up, but the number of patients with hemophilia is limited.15 Comparing birth cohorts from centers in 2 countries provides the best alternative to a randomized controlled trial to assess long-term outcomes of the Dutch intermediate-dose and Swedish high-dose prophylactic regimens. Selection bias was avoided, as the choice of prophylactic regimen depended on country of birth only. In addition, external factors such as social circumstances and level of general health care provision in Sweden and the Netherlands are quite similar.
The aim of this study was to compare long-term outcomes and costs between the Dutch intermediate-dose and the Swedish high-dose prophylactic regimens for persons with severe hemophilia with a follow-up of up to 3 decades. As optimal dosing for prophylaxis has never been established, this study provides a unique insight that could not have been reported previously.
Methods
Design and setting
The study was designed as an observational study comparing 2 cohorts, using retrospective assessment of treatment and prospective assessment of outcome. The study was performed at the hemophilia treatment centers of the University Medical Center Utrecht, the Netherlands (Van Creveldkliniek); the Karolinska University Hospital in Stockholm, Sweden; and the Skåne University Hospital in Malmö, Sweden. These clinics had routinely collected annual data on treatment and bleeding, hospital admissions, and surgical procedures for decades. Data for this study were collected between January 2006 and July 2009. Ethical approval for this study was obtained from the institutional review boards of Utrecht (nr 06-002) and Malmö (nr 413/2006 and 493/2007). This study was conducted in accordance with the Declaration of Helsinki.
Patients
All patients with severe hemophilia (FVIII/IX < 1% or < 1 IU/dL) born between January 1, 1970, and January 1, 1994, who were treated at the participating centers and who had lifelong access to care and treatment data available were eligible for this study. Patients with a history of inhibitors (any inhibitor activity > 0.6 Bethesda Units with decreased recovery) were excluded. Assessments were performed during regular outpatient visits. Patients aged 18 years and older were considered as adults. Informed consent was obtained from all patients before participation.
Patient characteristics and treatment history
Baseline patient characteristics registered included date of birth, date of diagnosis, type of hemophilia, hepatitis C status and HIV status, and date of first joint bleed.
To assess treatment history, date of first treatment, start of home treatment, and onset of prophylaxis, as well as complete history of prophylactic regimens used, were collected. In addition, orthopedic surgical procedures (including arthroscopies and radioactive synovectomies) were extracted from patient files.
Current treatment
For the last 5 years before evaluation, annual clotting factor consumption was extracted from patient logs and hospital pharmacy records. In addition, the number of visits to the center and details on hospital admissions was documented.
Outcome
The primary outcome parameter was clinical joint status, assessed by the center’s physiotherapist, using the Haemophilia Joint Health Score (HJHS; version 1.0).16,17 The HJHS is based on physical examination of elbows, knees, and ankles (maximum, 20-26 points per joint) and observation of gait for knees and ankles (0-4 points). The total score was calculated without adding overall global gait to the individual joint scores, resulting in a total score ranging from 0, signifying perfect joint health, to 144. The HJHS score was originally developed to assess subtle joint damage in children with hemophilia. The score was used for this study because the items scored are not age-specific and because differences in outcome were expected to be small. All HJHS scores were performed by a single physiotherapist at each participating center. Standardization and reliability were established during a training session (January 2006; 12 patients; intraclass correlation, 0.84) with all 3 designated physiotherapists.18
Secondary outcome parameters were the annual number of joint bleeds, self-reported activities, health-related quality of life, and social participation. The annual number of joint and soft tissue bleeds during the last 5 years was extracted from the patient logs, medical files, and hospital databases by research nurses at each center. Bleeds were defined as any complaint requiring treatment with clotting factor concentrate. Bleeds located in shoulders, elbows, wrists, hips, knees, or ankles were considered joint bleeds. All data were entered in an electronic case report form, using predefined definitions. To minimize bias, all definitions and how to complete the electronic case report form were documented and discussed before the study start.
Questionnaires were administered to adult patients only. Self-reported limitations in activities were assessed using the Haemophilia Activities lists,19-21 whereas physical activity levels were assessed by the International Physical Activity Questionnaire.22 Health-related quality of life expressed as utility was assessed using the Euroqol (EQ-5D).23 EQ-5D utility values were calculated using the Dutch tariff24 for both cohorts.
To compare social participation, data on achieved level of education and labor market participation were collected and compared with data for the age-matched general male population in the respective countries, using the Labor Force Survey at Statistics Netherlands25 and Statistics Sweden,26 respectively, and the Swedish Registration of Education.27
Cost and resource use
Dutch prices for the year 2010 were collected for evaluation of health care resource use and lost production in both cohorts. Prices were based on national price lists28,29 and on academic hospital prices from 2011 for surgeries (Table 1). Days lost from work were valued according to the human capital approach.30 Costs were translated to US dollars using the European Central Bank 2010 bilateral average annual exchange rate: €1 = US$1.3257 (http://sdw.ecb.europa.eu).
Direct medical costs (factor concentrate costs and other costs) and indirect costs (cost of days lost from work) for the 5-year evaluation period were compared between cohorts.
In addition, lifelong use of factor concentrates according to age and treatment strategy was estimated from individual-level data on the history of prescribed prophylactic regimens and body weight for Swedish patients and from an earlier study for Dutch patients.31 Factor consumption according to age and treatment strategy was also compared graphically.
Statistics
Student t tests, nonparametrical Mann–Whitney U tests, and χ-squared tests were used to compare patient characteristics and outcomes according to treatment strategy. Panel data population-averaged generalized linear regression was used to predict the average annual cost of a mean-weight adult patient for each treatment regimen. Both logistic analysis (dependent HJHS ≥ 10) and generalized linear models (dependent HJHS, γ distribution, log link) were used to study the effects of age at start of prophylaxis independent of country, age at evaluation, and 5-year factor consumption. Statistical analyses were performed using SPSS version 20 (IBM Corp., Armonk, NY) and Stata version12 (StataCorp LP, College Station, TX).
Results
Patients
Seventy-eight Dutch (intermediate-dose) and 50 Swedish (high-dose) patients were assessed during regular outpatient visits. The overall inclusion rate was 128/156 (78%), including 78/92 (85%) Dutch patients (8 refusals, 5 unable to include because of irregular visits, and 1 patient not invited as he was currently taking interferon) and 50/71 (70%) Swedish patients (21 refusals).
To assess the effect of excluded patients on the overall study population, we compared age, previous orthopedic surgeries, and treatment with clotting factor concentrate during the last 5 years between excluded and included patients for both countries. Dutch excluded patients (n = 14) were significantly older (mean age, 32.3 vs 24.9 years; P < .01) but had a similar history of previous orthopedic surgery (21% vs 15%; P = .69); excluded patients showed a trend toward using full prophylaxis less often (64% vs 78%; P = .31) and a 23% lower annual clotting factor consumption (mean, 1680 IU/kg per year; mean difference, 500 IU/kg per year; P = .06). Swedish excluded patients (n = 21) had a similar age (25.9 vs 23.8 years; P = .24) and history of orthopedic surgery (5% vs 8%; P = 1.0) but displayed a trend toward using full prophylaxis less often (76% vs 86%; P = .15) and had a 21% lower annual clotting factor consumption (mean, 3240 IU/kg per year; mean difference, 865 IU/kg per year; P = .03)
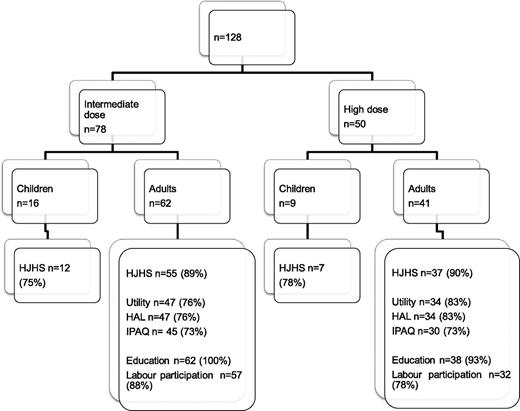
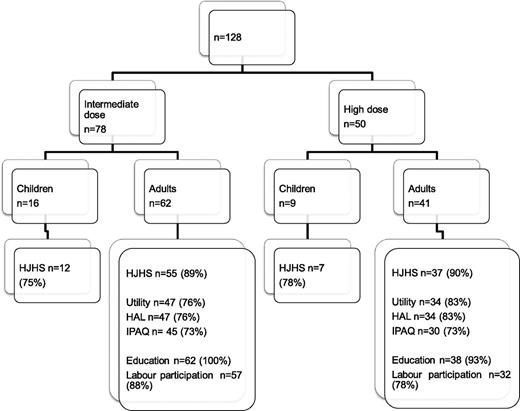
The majority of included patients were adults at the time of evaluation (Netherlands, 62 of 78 [79%]; Sweden, 41 of 50 [82%]). The number of patients with available data according to outcome parameter is shown in Figure 1.
Patient characteristics and treatment
The mean age of included patients was 24.5 years (range, 14-37 years).The majority (115 of 128; 90%) of patients had hemophilia A. Overall, 34% of patients were positive for hepatitis C, and 5% were HIV-positive. Although the prevalence of HIV was similar, hepatitis C infection was more common in Dutch patients (42% vs 22%; P = .04).
Patient characteristics and treatment according to prophylactic regimen are shown in Table 2. Patients were diagnosed with severe hemophilia early in life in both countries (median, 0.7 years; IQR, 0.2-1.0 years). Dutch patients entered the clinic about 1 year later than Swedish patients, at a median age of 1.8 years versus 0.6 years (P < .01). Treatment was started early in both countries. The first infusion was usually given around the age of 1 year, but at a slightly older age in Dutch patients (median, 1.1 years vs 0.9 years; P value < .01). Patient characteristics and treatment in the 2 Swedish centers were comparable.
Both cohorts included a single patient with a mild bleeding phenotype who never started prophylactic treatment: a Dutch patient born in 1972 and a Swedish patient born in 1979. Overall, the prophylactic treatment regimens were very different: Patients treated with the Dutch intermediate-dose regimen started prophylaxis later, mostly after the onset of joint bleeding, and switched to home treatment at a later age. Since the start of prophylaxis, most patients continued this treatment, although there was a trend toward more frequent discontinuation (P = .19) and a significantly lower proportion of Dutch patients receiving full-time prophylaxis during the last 5 years (78% vs 96%; P < .01).
At evaluation, the overall annual consumption was 2150 IU/kg per year (95% CI, 1600-2700) lower for the intermediate-dose regimen (median, 2100 vs 4000 IU/kg per year; P < .01). The frequency of infusions was similar, at around 3 infusions per week. Patient characteristics and treatment patterns were similar for hemophilia A and B (data on request).
Clinical outcome
Clinical outcome according to regimen is shown in Table 3. In total, 643 patient-years were evaluated for bleeding and treatment. Overall, physical activity was high in both groups and bleeding frequencies were low. When comparing both regimens, however, the intermediate-dose regimen resulted in a limited but statistically significant increase in the number of bleeds, at just more than 1 additional joint bleed per year (median, 1.3 vs 0 bleeds; P < .01) and 7 to 8 additional bleeds (median, 10 vs 2.5 bleeds) over the course of 5 years. During the 5-year observation period, a single Dutch HIV-positive patient experienced an intracranial bleed. No other life-threatening bleeds were observed in either group.
In these young adults, only minor changes in joint status were observed, and few limitations in activities were reported. However, again, in the direct comparison, the patients treated with the intermediate-dose regimen had slightly, but significantly, higher HJHS scores (median, 9.0 vs 7.0 points of 144) and reported slightly, but significantly, more limitations in daily activities (median Haemophilia Activities List score, 93 vs 99 of 100). However, high-dose prophylaxis did not completely prevent joint damage in all patients: 5/44 (11%) of Swedish patients still had a HJHS of 10 or more points compared with 31/68 (46%) of Dutch patients (P < .01). A history of orthopedic surgery was rare in both populations, at 15% of Dutch patients compared with 8% of Swedish patients (P = .29). Regression analyses did not show an independent effect of age at start of prophylaxis on outcome (β = 0.098 [P = .21] for logistic regression and β = 0.032 [P = .18] for generalized linear models). Outcome parameters were similar for hemophilia A and B (data on request).
Quality of life
The quality of life measured by EQ-5D utility was high (Table 3) and was similar across both cohorts (P = .93). At the group level, values were close to those of the general male population aged 20 to 29 years: mean utility was 0.88 for Dutch patients vs 0.93 in the Dutch general population,32 and 0.86 for Swedish patients vs 0.91 in the Swedish general population.33
The response rate was lower for Dutch patients (76% vs 83%; Figure 1), but this is not expected to have affected outcome, as nonresponders and responders had similar ages and education and employment levels, as well as joint status.
Social participation
The achieved level of education and labor force participation rates for adult patients are shown in Table 4. At evaluation, a higher percentage of Swedish participants reported having completed university education, but the differences in overall educational achievement were not significant. Compared with the general male population, fewer participants had achieved a university degree at evaluation, although this may result from some still being students and national statistics not including the youngest adults (The Netherlands, age 25+ years; Sweden, 20+ years).25,27
Among employed participants, full-time employment dominated: 38/43 (88%) of Dutch and 26/30 (87%) of Swedish patients were working full-time. Few patients were unemployed (The Netherlands, 2; Sweden, 4), and the unemployment rates among participants were similar (The Netherlands) or lower (Sweden) than those of their peers.
Overall, 86% of patients receiving either regimen did not report any days lost from work or school because of hemophilia during the 5-year study period. Among patients who reported missing days from work or education on a short- or long-term basis, the median number of days lost during the 5-year period for Dutch patients (n = 11) was 202 (IQR, 39-536) days compared with 28 (IQR, 3-39) days for Swedish patients (n = 7). This large difference was driven by 5 Dutch patients: 3 patients undergoing interferon-based treatment of HCV infection who could not work for on average 7.7 months and 2 who were disabled on a long-term basis, 1 after an intracranial bleed and another for severe arthropathy, HIV, and HCV infection.
Costs
The mean and median 5-year treatment costs in US$1000 per patient according to prophylactic regimen and Dutch prices are shown in Table 5. For the 5-year period, median total costs per patient were 73% higher for high-dose prophylaxis: US$0.85 million (IQR, US$0.66-US$1.09 million) for Dutch vs US$1.48 million (IQR, US$1.15-US$1.79 million; P < .01) for Swedish patients. During this period, the clotting factor consumption dominated costs, making up 97.1% of costs for the intermediate-dose regimen and 99.6% for the high-dose regimen. On average, resource use and costs were 40% to 50% lower for the intermediate-dose regimen, except for a US$700 higher category for other health care (including surgery, hospitalizations, and health care visits), which accounted for less than 1% of total costs. Using 5-year data, the cost per bleed avoided would be US$91 000.
The predicted annual total costs for an average-weight patient (74 kg) were 66% higher for high-dose prophylaxis: mean US$179 600 per year (95% CI, US$163 000-US$196 200) for Dutch patients versus US$297 900 per year (95% CI, US$270 800-US$324 900) for Swedish patients (P < .01).
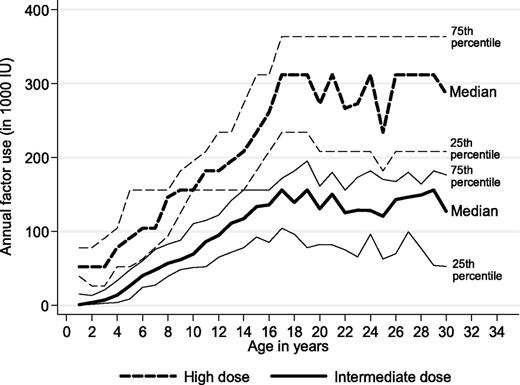
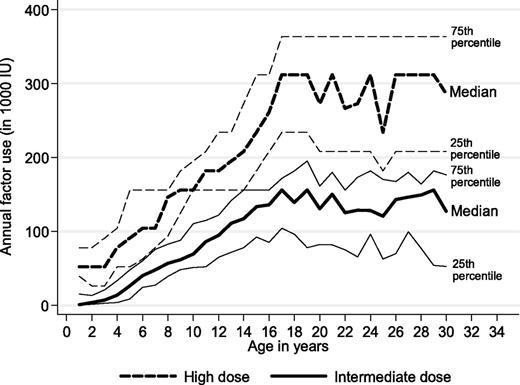
A clinically and statistically significant difference in factor concentrate use between the 2 prophylactic regimens was also seen in a 30-year perspective (P < .01 all ages; Figure 2). Absolute differences in median annual factor use increased with age, ranging from around 50 000 IU per year before school age to 100 000 IU per year at age 15 years and to 150 000 IU per year at age 20 to 30 years. Extending the time horizon to a 70-year-perspective, lifetime factor use per patient would amount to 8.2 versus 18 million IU, assuming constant factor concentrate dosages at current median levels for adults on both regimens. At a price of US$1.10 per IU, these differences are equivalent to a lifetime cost of factor concentrate of US$9.1 versus US$20 million (undiscounted) or US$3.3 versus US$7.1 million (discounted by 3%).
Observed annual factor use for persons with severe hemophilia receiving high-dose and intermediate-dose prophylaxis (Mann-Whitney all ages P < .001).
Observed annual factor use for persons with severe hemophilia receiving high-dose and intermediate-dose prophylaxis (Mann-Whitney all ages P < .001).
Discussion
This study is the first prospective study comparing the outcomes of 2 different prophylactic treatment regimens for patients with severe hemophilia with 20 to 30 years of follow-up.
Outcome was favorable in the majority of patients. At a median age of 24 years, 54% of those receiving intermediate-dose prophylaxis and 89% of those receiving high-dose prophylaxis had no significant arthropathy (HJHS score < 10/144; P < .01); quality of life and employment status were similar. Because of differences in clotting factor consumption, costs diverged widely during the last decades. Annual total costs for a 74-kg patient were 66% higher for high-dose prophylaxis (mean, US$179 600 per year for Dutch versus US$297 900 per year for Swedish patients).
Over the course of 5 years, the high-dose regimen was associated with a 73% higher total costs (median, US$0.85 million for Dutch vs US$1.48 million for Swedish patients).
Study design
To analyze the long-term outcomes of prophylactic treatment regimens, we used a combination of state-of-the-art prospective outcome assessment34 and collection of retrospective data from patient files. Use of routinely documented data enabled extraction of bleeding and full treatment history; questionnaires were validated, and every effort was undertaken to standardize joint assessment. Similar to a randomized controlled trial, the prophylactic regimen was allocated on the basis of study group (ie, country of birth) and not on clinical characteristics or ability to pay for treatment. The study aimed to compare full birth cohorts to avoid selection bias. Key treatment characteristics of nonparticipants were analyzed. In both countries, nonparticipants showed a trend toward less intensive treatment with a lower annual consumption of factor concentrates (intermediate-dose regimen, –23%; high-dose regimen, –21%). This implies that the results of this study slightly overestimated annual total costs, but it is unlikely to have biased the results of the cost comparison. The effects on outcome cannot be estimated: nonparticipants may have a milder bleeding pattern and/or a lower adherence to prophylaxis.
Although it is common to report clinical results separately for hemophilia A and B, this distinction may be less important for the aim of this article. As expected, the differences between prophylactic regimens were consistent when restricting the analysis to persons with hemophilia A only.
Comparison with other studies
Earlier studies have covered long-term clinical outcomes of prophylactic treatment of Dutch and Swedish cohorts.3,5 A previous retrospective comparison of Dutch and Swedish adolescent patients, lacking formal outcome assessment and collection of cost data, observed statistically significant differences in joint outcome among patients younger than 15 years only.35 Other studies performed comparisons of high-dose prophylaxis to on-demand treatment in young patients aged up to 66 and 117 years, respectively. The lack of long-term controlled studies was recently considered an important limitation of economic evaluations.36
When considering the incremental cost of providing lifelong prophylaxis instead of on-demand treatment, there are very few data available. So far, only 2 studies have presented cost data in unselected cohorts of patients with severe hemophilia who were treated on demand. At the price levels from 2000, mean direct medical costs of €79 000 per year (US$73 000) were reported in France37 and €52 000 per year (US$48 000) in Norway.10 Quality of life was not measured in these studies. At this time, the Hemophilia Utilization Group Study is collecting cost data in the United States.38 It is evident that on-demand treatment is much less costly than the prophylactic regimens presented in this study. However, the long-term outcome of on-demand treatment is worse in terms of joint status, serious hemorrhages, quality of life, and labor force participation.4,37,39
Previous health and economic evaluations have addressed issues of cost-effectiveness in hemophilia care.9,40 One cost-benefit analysis in a Swedish setting reported that the average willingness to pay for prophylaxis in the general population exceeded the average cost of provision of prophylaxis per taxpayer.41 This study does not contain a formal economic evaluation, as the cost differences were nearly US$159 000 per year and the difference in utility was not statistically significant in these data. Using 5-year data, the cost per bleed avoided would be US$120 600. Therefore, any cost-utility analysis on these data will show that compared with intermediate-dose prophylaxis, general provision of high-dose prophylaxis will not be cost-effective at current prices and cost-effectiveness thresholds (eg, €80 000 or US$106 056 per quality-adjusted life-year in the Netherlands).
Clinical implications
This study shows a statistically significant but small incremental benefit after nearly doubling the annual prophylactic dose. The benefit was observed in all outcome parameters except quality of life. This may reflect the limited clinical effects of an additional joint bleed per year or the inability of the generic EQ-5D questionnaire to pick up small differences. From a lifelong perspective, it is expected that differences in outcome between these 2 cohorts will have increased in another 20 years. However, we do not know the extent or the clinical implications of such an increase.
Is the difference attributable to dose difference only? One of the drivers of the slightly better outcome in the high-dose group may be the earlier start of prophylaxis. This is well-established,42,43 and both countries have started prophylaxis earlier during the last decades.2,3 Regression analyses failed to identify a statistically significant and independent effect of age at start of prophylaxis in outcome. This unexpected finding may be a result of 2 limitations in the present data: lack of variation and limited power. Lack of variation was present in the Swedish data, as all patients started prophylaxis very early. Power was limited by small differences in outcome and limited patient numbers.
At this time, prophylactic dosing is mostly based on the Swedish regimen of 25 to 40 IU/kg per infusion, and dosages used in pediatric trials have been consistently high, at 25 IU/kg thrice weekly or every other day (ie, 3900 and 4550 IU/kg per year, respectively).6,7 For older patients, guidelines on dosing are unavailable,44,45 and the recommendation is to just keep this dose,45 despite the fact that adults have more regulated activity patterns, a longer FVIII half-life, and a weaker association between trough levels and bleeding.46,47
For clinical practice, it will always be important to prevent bleeding, especially in joints. Overall, these favorable results support the need for an early start of prophylaxis and continuing this treatment in adults with severe hemophilia. At patient level, the data on joint outcome suggest that a proportion of patients are equally well-off with intermediate-dose prophylaxis, whereas others need a high-dose regimen to control their bleeding. In the absence of valid laboratory parameters to assess a patients’ phenotype, clinical parameters of bleeding frequency and physical activities, combined with pharmacokinetic information,48 are the only tools available to individualize prophylactic dosing. Eventually, some adult patients even discontinued prophylaxis without experiencing frequent bleeding, as was observed in these and other cohorts.49
In conclusion, this first direct comparison of 2 prophylactic regimens suggests that at a group level, a more intensive and higher-dosed regimen may provide slightly improved outcome at a significant cost increase. At the patient level, the challenge is to identify patients who will be as well-off receiving lower doses without compromising patient safety. Even in small patient groups such as these, improving the cost-effectiveness of treatment should be considered.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a Bayer Haemophilia Award (special project 2005).
Authorship
Contribution: K.F., K.S.C., P.P., M.H., R.L., H.M.v.d.B. and E.B. conceived the study; K.F., P.P., K.S.C., H.M.v.d.B., and E.B. designed the study; K.F. and E.B. secured funding; K.F., H.M.v.d.B., and E.B. managed all study procedures (ethics and governance, recruitment, patient assessment, data management); K.F. and K.S.C. planned and undertook the statistical analysis and drafted the manuscript with input from all authors; all authors had access to the data and analysis and approved the final manuscript; and K.F. is the guarantor.
Conflict-of-interest disclosure: All authors are independent of the funding source. K.F. has acted as a consultant and participated in expert groups for Bayer, Baxter, Biogen, and Novo Nordisk, has received research grants from Baxter, Novo Nordisk, Pfizer, and CSL Behring, has given lectures for Bayer, Baxter, Novo Nordisk, and Pfizer, and has received travel support from Baxter. K.S.C. has acted as a consultant for Baxter, has received research grants from LIF, and has given lectures for Bayer. P.P. has participated in an advisory board for Pfizer, has given lectures for Bayer, Baxter, and Pfizer, and has received travel support from Bayer, Baxter, and Pfizer. M.H. has received research grants from Octapharma and Baxter, and has given lectures for Baxter, Bayer, CSL Behring, and Leo-Pharma. R.L. has acted as a consultant and participated in expert groups for Bayer and Novo Nordisk, has received research grants from Baxter, and has given lectures for Bayer, Baxter, and Novo Nordisk. H.M.v.d.B. has received unrestricted funding from Baxter, Bayer, and Novo Nordisk, has acted as a consultant for Bayer, and has given lectures for Bayer and Baxter. E.B. has acted as a consultant and participated in expert groups for Bayer, Baxter, Novo Nordisk, Sobi, Octapharma, and CSL-Behring, and has given lectures for Bayer, Baxter, Novo Nordisk, Pfizer, Octapharma, Sobi, and CSL Behring.
Correspondence: Kathelijn Fischer, University Medical Center Utrecht, Van Creveldkliniek, Room C01.425, PO Box 85500, 3508 GA Utrecht, The Netherlands; e-mail: k.fischer@umcutrecht.nl.