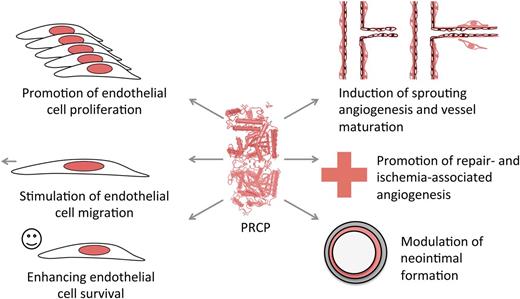
In this issue of Blood, Adams et al provide evidence for an important novel function of prolylcarboxypeptidase (PRCP), or angiotensinase C, in endothelial cell physiology and angiogenesis.1
Angiogenesis, the process by which new blood vessels are generated during development, has a critical role in solid tumor progression and hematological malignancies.2 Understanding how druggable proteins regulate this complex process is crucial for the development of new pharmacological leads for anticancer therapy.
The PRCP gene encodes for a serine protease that generates plasma kallikrein from prekallikrein and degrades angiotensin II and α-melanocyte-stimulating hormone (α-MSH) by proteolyzing their C-terminal Pro-X bonds.3,4 PRCP also was recognized to inactivate α-MSH1-13’s ability to stimulate the melanocortin 4 receptor to induce anorexia by proteolyzing its C-terminal Pro-Val bond.5 In vivo loss of function of PRCP in gene trap (PRCPgt/gt) mice confirms that a major role of this protein is in metabolism because these mice have significantly reduced body weight.5 In fact, PRCPgt/gt mice have better glucose tolerance and less insulin resistance than their wild-type controls.6 These observations were used to control food intake, with encouraging results: a selective PRCP inhibitor termed UM8190 induces murine anorexia.7 It is of further interest that the peptide product angiotensin-(1-7) of PRCP protease action also promotes adipogenesis.8 This latter observation suggests an autocrine function of PRCP action in fat metabolism.
A role for PRCP in vascular development has been suspected, because its transcripts are up-regulated during an active growth phase of the chick extra-embryonic vasculature,9 it is overexpressed in the endothelium,9 and the protein is present at surface of endothelial cells.3
A series of experiments that investigated the role of PRCP in blood pressure and coagulation control showed that, even in the presence of metabolic health, PRCPgt/gt mice are hypertensive and prothrombotic.10 These animals have vascular inflammation with reduced Kruppel-like factors 2 and 4, uncoupled nitric oxide, and reduced protein C activation due to dysfunctional thrombomodulin. The overall mechanistic basis for these findings in PRCPgt/gt vessels is increased reactive oxygen species that is corrected by antioxidants.10
Do these observations in the PRCPgt/gt mice transfer to vascular activities? Does PRCP also control angiogenesis? In a series of classical in vitro and in vivo angiogenesis assays, Adams et al show that PRCP has widespread effects on endothelial cell function (see figure).1 In vitro, it influences endothelial cell division, a major process, which is shared with established endothelial growth factors such as vascular endothelial growth factor or fibroblast growth factor-2. This finding is a rather unexpected effect of this protein and, more interestingly, is independent of its enzymatic activity, because a PRCP construct with a loss-of-function mutation in its active site conserves biological activity on endothelial cells. Like the serine proteases factor XII and urokinase and tissue type plasminogen activators, PRCP appears to have both proteolytic and nonproteolytic functions. Furthermore, endothelial cell motility and survival are both enhanced by PRCP, showing that this protein acts like an endothelial growth factor. These in vitro results have been confirmed by more sophisticated models such as the mouse aortic ring assay, which recapitulates major steps of sprouting angiogenesis and the matrigel plug assay, which measures development of growth factor–induced capillaries. Both assays have been performed in PRCPgt/gt mice. In the plug assay, reduced PRCP levels were also associated with a defect in vessel maturation because newly formed capillaries have reduced pericyte coverage. A significant decrease in angiogenesis was also observed in skin wounds of PRCPgt/gt mice, resulting in delayed wound closure. Accordingly, in a limb ischemia model, a reduced angiogenic response was found in these mice, as evidenced by reduced blood flow and vascular density. In a femoral artery wire injury model where endothelial cells of a large vessel were detached from the underlying vessel structure, lack of PRCP causes neo-intimal thickening, probably due to delayed re-endothelization, enhanced smooth muscle cell proliferation, and recruitment of CD45+ inflammatory cells. Vascular inflammation produced by PRCP deficiency is ameliorated by an interaction between PRCP and MRP-14, a protein that mediates leukocyte migration. Doubly deficient PRCPgt/gt/MRP-14−/− mice restore the inflammatory response to that like wild-type control mice in this model.
The obvious questions raised by this study are numerous. Are PRCP actions on the endothelium mediated through a specific receptor? What intracellular signaling pathways are controlled by the nonenzymatic properties of PRCP? Will it be possible to design small molecule inhibitors of PRCP that specifically block these actions? Another challenging question is how efficient PRCP inhibitors will be in tumor angiogenesis. Preclinical studies should be performed to access the safety and efficacy of small molecule PRCP inhibitors in such settings, with a special emphasis on the modulation of the immune response. Last, it may be worth trying to chemically couple PRCP inhibitors to vascular-targeting agents to limit possible side effects in other tissues. Taken together, the results of Adams et al suggest that PRCP is a very promising target to control processes in which pathological angiogenesis plays an important role.
Conflict-of-interest disclosure: The author declares no competing financial interests.