In this issue of Blood, Wilkinson and Pantopoulos show that iron regulatory protein 1 (IRP1) regulates erythropoiesis and systemic iron homeostasis in mice by controlling hypoxia-inducible factor 2α (HIF-2α) messenger RNA (mRNA) translation.1
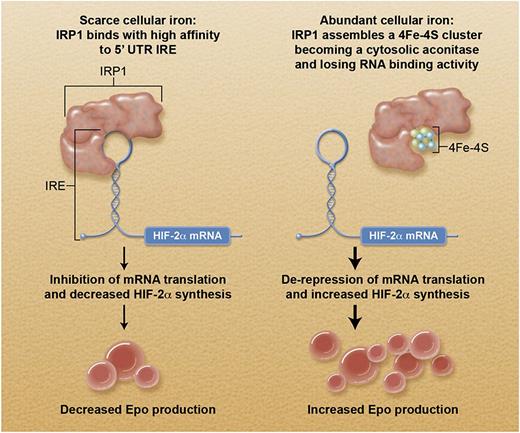
Schematic representation of how cellular iron status controls erythropoietin production through the IRP1-HIF-2α axis. In this scheme, the cell of interest is a specialized interstitial renal fibroblast that responds to tissue hypoxia by producing erythropoietin. The IRP1/IRE-mediated translational control primarily influences HIF-2α synthesis: the final impact on erythropoietin production depends also on oxygen tension (see text). Professional illustration by Alice Y. Chen.
Schematic representation of how cellular iron status controls erythropoietin production through the IRP1-HIF-2α axis. In this scheme, the cell of interest is a specialized interstitial renal fibroblast that responds to tissue hypoxia by producing erythropoietin. The IRP1/IRE-mediated translational control primarily influences HIF-2α synthesis: the final impact on erythropoietin production depends also on oxygen tension (see text). Professional illustration by Alice Y. Chen.
Earlier this year, 2 papers in Cell Metabolism2,3 reported studies supporting a central role of the IRP1–HIF-2α axis in coordinating iron metabolism and oxygen sensing. Altogether, these 3 papers establish a molecular link between cellular iron status and erythropoietin production.
Most of body iron is found in circulating red cells as an essential component of hemoglobin, and most of internal iron exchange cycles through the erythron. A strict coordination of oxygen sensing, erythropoiesis, and iron metabolism is indispensable, and this regulation has been finely tuned during evolution.
Erythropoietin is the primary regulator of erythropoiesis and exerts its effects by binding to a surface receptor present on erythroid progenitors and by preventing their apoptosis. In turn, erythropoietin production is primarily regulated by tissue oxygenation through a mechanism of oxygen sensing that involves HIF.4 HIF responds to changes in tissue oxygen concentration and acts as a transcription factor for the EPO gene. HIF is a heterodimer composed of 2 subunits: α and β. Whereas the latter is constitutively expressed, the α subunit is inducible by hypoxia. When cellular oxygen tension is normal, the α subunit is prolyl-hydroxylated, binds the von Hippel-Lindau (VHL) protein, and is ubiquitinated and degraded through the proteasome. When cellular oxygenation is low, prolyl-hydroxylation is less efficient, and the α subunit is stabilized: it binds to the β subunit, and forms an efficient HIF transcription factor. The increased EPO transcription involves increased erythropoietin synthesis, and this in turn leads to expansion of erythropoiesis.
Three HIF-α isoforms have been identified: HIF-1α, HIF-2α, and HIF-3α. In a study of familial erythrocytosis, Percy et al5 provided evidence that HIF-2α, encoded by the HIF2A gene, is the main transcription factor that regulates erythropoietin levels in humans. A few years ago, Sanchez et al6 reported the discovery of a conserved, functional iron-responsive element (IRE) in the 5′ untranslated region (UTR) of the mRNA encoding HIF-2α. IREs are mRNA stem-loop structures capable of interacting with mRNA-binding proteins, known as IRPs.7 In mammalian cells, coordinated regulation of transferrin receptor 1 (TfR1) and ferritin synthesis is operated by IREs and by IRP1 and IRP2 at the level of mRNA translation.7 One IRE is present in the 5′ UTR of H- and L-ferritin mRNA, whereas multiple IREs are present in the 3′ UTR of TfR1 mRNA. When cellular iron is scarce, ferritin synthesis is inhibited, and TfR1 expression is increased; the opposite occurs when cellular iron is abundant.7 Hereditary hyperferritinemia with or without cataract (Online Mendelian Inheritance in Man #600886) is an autosomal dominant disorder caused by heterozygous mutation in the 5′ UTR IRE of the L-ferritin mRNA.7
The study by Wilkinson and Pantopoulos demonstrates that IRP1 specifically regulates HIF-2α mRNA translation and HIF-2α–dependent physiological responses in mice. In detail, deletion of IRP1 caused translational de-repression of HIF-2α, overproduction of erythropoietin, reticulocytosis, and polycythemia. Juvenile IRP1-null (Irp1−/−) mice had evidence of suppressed hepcidin expression, with elevated serum iron and iron depletion in splenic macrophages due to unrestricted expression of ferroportin. Interestingly, hematologic abnormalities of Irp1−/− mice appeared to be corrected by age, suggesting that compensatory mechanisms might neutralize the effects of the lack of IRP1 over time.
The figure illustrates the pathophysiological implications of the novel molecular link between cellular iron and erythropoiesis. When cellular iron is scarce (eg, depletion of body iron stores), inhibition of HIF-2α mRNA translation likely contributes to limiting erythropoiesis to save iron for other essential biological reactions. Similarly, this mechanism may be useful in patients with erythrocytosis. In iron deficiency anemia, because HIF-2α levels also depend on oxygen tension, hypoxia promotes stabilization of HIF-2α, likely compensating for its defective synthesis. When cellular iron is abundant, the impact of HIF-2α mRNA translation on HIF-2α levels depends on oxygen tension. Under normoxic conditions (eg, a patient with genetic hemochromatosis), the excess HIF-2α is targeted for degradation through the proteasome. By contrast, under hypoxic conditions (eg, a patient with thalassemia major), inhibition of prolyl-hydroxylation promotes stabilization of the excess HIF-2α, leading to overproduction of erythropoietin.
Is there any possibility that disruption of the IRP1–HIF-2α axis is responsible for the molecular pathogenesis of human disease? Ghosh et al2 previously described pulmonary hypertension in the Irp1−/− mice they generated. This manifestation was likely a result of overexpression of HIF-2α in pulmonary endothelial cells, which in turn induced high expression of endothelin-1. That pulmonary hypertension may derive from disruption of the IRP1–HIF-2α axis has been also demonstrated by a recent report on erythrocytosis and pulmonary hypertension in a mouse model of human HIF2A gain-of-function mutation.8 Somatic gain-of-function mutations of HIF2A have been identified in patients with paraganglioma with polycythemia.9 Hereditary hyperferritinemia with or without cataract might be just the first of a series of inherited disorders associated with mutant IREs. In fact, heterozygous mutations in the 5′ UTR IRE of HIF-2α mRNA would prevent binding of IRP1, enhance HIF-2α translation and erythropoietin production, and in turn lead to erythrocytosis. Finally, in a mouse model with inducible gain of IRP1 function, IRP1 activation has been found to cause macrocytic anemia.10 Therefore, because of the discovery of the IRP1–HIF-2α axis, the chapter of translational pathophysiology may soon expand.7
Conflict-of-interest disclosure. The author declares no competing financial interest.