Key Points
Chemically modified nonanticoagulant heparins are strong inhibitors of hepcidin expression in normal and Bmp6−/− mice.
These heparins abolish hepcidin induction caused by LPS, a model of inflammation, and are candidates for treatment of inflammatory anemia.
Abstract
Hepcidin controls systemic iron availability, and its excess contributes to the anemia of chronic diseases, the most prevalent anemia in hospitalized patients. We previously reported that heparins are efficient hepcidin inhibitors both in vitro and in vivo, but their anticoagulant activity limits therapeutic use. We studied nonanticoagulant heparins produced by N-acetylation and oxidation/reduction (glycol-split) that lost antithrombin-binding affinity. Four nonanticoagulant heparins inhibited hepcidin expression in hepatic HepG2 cells and primary hepatocytes. The 2 most potent ones used in mice suppressed liver hepcidin expression and serum hepcidin in 6 hours, with a significant decrease of spleen iron. This occurred also in lipopolysaccharide (LPS)-treated animals that mimic inflammation, as well as after chronic 1-week treatments, without evident adverse effects on coagulation. Heparin injections increased iron mobilization and facilitated the recovery from the anemia induced by heat-killed Brucella abortus, a model of inflammatory anemia. The heparins were used also in Bmp6−/− mice. A single dose of heparin reduced the already low level of hepcidin of these mice and prevented its induction by LPS. These nonanticoagulant compounds impair bone morphogenetic protein /sons of mothers against decapentaplegic signaling with no evident adverse effect in vivo, even when administered chronically. They may offer a strategy for the treatment of diseases with high hepcidin levels.
Introduction
Iron is essential for many vital functions, but it is also potentially toxic and needs tight regulation.1-3 In mammals, the cellular control relies mainly on the iron regulatory proteins,4-7 whereas at a systemic level, control is mainly achieved through the modulation of the iron exporter ferroportin,8,9 whose functionality is mainly modulated by hepcidin, a liver peptide hormone that binds the exporter and induces its degradation.10,11 Thus, hepcidin is a key regulator of systemic iron,12 and its expression is regulated by several factors, including tissue and serum iron availability, hypoxia, erythroid activity, and inflammation.13 Deregulation of hepcidin expression occurs in genetic disorders, including hemochromatosis and iron refractory iron deficiency anemia, as well as in many acquired conditions, the most common of which is the anemia of chronic disease, also known as anemia of inflammation.14
Liver hepcidin expression is controlled primarily by the bone morphogenetic protein (BMP)/sons of mothers against decapentaplegic (SMAD) signaling pathway.15,16 Hemojuvelin (HJV) acts as a BMP coreceptor15,17,18 and its modulation by proteases participates in the control of hepcidin expression.18 BMP6 seems to be the main physiological regulator of hepcidin expression in vivo.19 BMP2 and BMP4 with strong osteogenic activity are also efficient inducers of hepcidin in vitro.20,21 Moreover, hepcidin is stimulated by inflammation through cytokines, mainly interleukin 6 (IL-6), in a pathway involving signal transducer and activator of transcription 3 (STAT3) phosphorylation,22,23 a signaling pathway induced also by Oncostatin-M24 and Genistein.25 Activin B regulates liver hepcidin expression in vivo by eliciting phosphorylation of SMAD1/5/8;26 it is stimulated by LPS and inflammation and, thus, cooperates with IL-6 for inducing hepcidin during the acute phase response.26
The development of hepcidin modulators for therapeutic use is attractive, and many approaches have been proposed (reviewed in Sun et al27 ). Among them, we showed that heparin is a strong inhibitor of hepcidin expression in hepatic cell lines, in mice, and possibly also in patients with high hepcidin levels.28 We proposed that heparin sequesters BMP6 and inhibits the BMP/SMAD activation.28 BMPs are heparin-binding molecules,29 and their osteogenic activity is modulated by exogenous heparins.30,31 Heparin inhibited BMP2 binding to its receptors,32 suppressed BMP7- and BMP6-mediated signaling,33 and modulated the activity of BMP2 and BMP4.34 Of note, a recent study has confirmed that heparin inhibits BMP6 activity and signaling in C2C12 myoblasts.35
Heparin anticoagulant activity is a result of high-affinity binding to antithrombin36 and is structurally similar to the heparan sulfates of proteoglycans present on various cell types, including hepatocytes.37 Heparan sulfates of proteoglycans are involved in many functions38 and are coreceptors for the activity of growth factors and cytokines.39 The osteogenicity of BMP2, BMP4, and BMP6 is dependent on heparan sulfates of proteoglycans.40 Thus, heparin capacity to interfere with BMPs signaling may be unrelated with anticoagulant activity, and heparins with little/no anticoagulant activity retain various biological activities such as antiheparanase,41 antiangiogenesis,42 and antiinflammation.43
Heparins with high antihepcidin activity and low anticoagulant activity may be useful for the treatment of disorders with hepcidin excess. Here we studied the functionality of chemically modified heparins with different degrees of sulfation, N-acetylation, and chain flexibility that lost antithrombin binding affinity and have a strongly reduced anticoagulant activity.44-46 We found that “reduced oxy-heparins,” alias “glycol-split” heparins (gs-heparins), have strong antihepcidin activity in vivo.
Methods
Heparins
The heparins were of mucosal origin and are listed in Table 1. One (Heparin) is the unfractionated commercial preparation previously used.28 Mucosal Heparin (MH) is a commercial unfractionated heparin with anticoagulant activity (API; Bioiberica lot F415-10/0001). The nonanticoagulant heparins included reduced oxyheparin (RO)-82 and RO-68, which were chemically modified by oxidation and reduction, NAc-91 modified by N-acetylation, and NAcRO-00 modified by both N-acetylation and oxidation/reduction, as described in Naggi et al.44 They have a different degree of sulfation (SO3/COO−), and RO-68 was partially 2O-desulfated before oxidation to obtain a higher number of gs residues for chain. All samples were recovered after desalting by freeze-drying and were characterized by nuclear magnetic resonance spectroscopy, conductometric determination of SO3/COO− ratio, and average molecular weight.
Cell culture and chemical treatment
The human HepG2 hepatoma cell lines (Lombardy and Emilia Romagna Experimental Zootechnic Institute) were cultured in minimum essential medium (PAA Laboratories GmbH), 10% endotoxin-free fetal bovine serum (Euroclone), 0.04 mg/mL gentamicin (Euroclone), 2 mM L-glutamine (PAA Laboratories GmbH), and 1 mM sodium pyruvate (Euroclone) and maintained at 37°C in 5% CO2. HepG2 cells (3 × 105 cells/well) were seeded onto 12-well plates, and after 24 hours, the medium was replaced with 1% fetal bovine serum. After 3 hours, the heparins were added with or without 10 ng/mL BMP6, 50 ng/mL IL-6 (ReliaTech GmbH), or 2 ng/mL Oncostatin-M (Sigma-Aldrich) for 6 or 16 hours.
Quantitative qRT-PCR
After the treatments, total cell RNA was recovered with TRI Reagent (Sigma-Aldrich), according to the manufacturer’s instruction. Reverse transcription was performed using 1 μg RNA, oligo dT, and Improm Reverse Transcriptase (Promega) in 20 μL. Samples of 1 to 2 μL were used for quantitative reverse-transcription polymerase chain reaction (qRT-PCR) assay, using iTAq Universal SYBR Green (Bio-Rad), according to the manufacturer’s instructions. Primers used for human cell lines were Hs Hamp forward, 5′-CCA-GCT-GGA-TGC-CCA-TGT-T-3′, and reverse, 5′-GCC-GCA-GCA-GAA-AAT-GCA-3′; Hs Hprt1 forward, 5′-TGC-TTT-CCT-TGG-TCA-GGC-AG-3′, and reverse, 5′-AAG-CTT-GCG-ACC-TTG-ACC-AT-3′; Hs Id1 forward, 5′-GTA-AAC-GTG-CTG-CTC-TAC-GAC-ATG-A-3′, and reverse, 5′-AGC-TCC-AAC-TGA-AGG-TCC-CTG-A-3′. The same procedure was used for mouse liver and the primers for quantitative real-time RT-PCR assay were: Mm Hamp1 forward, 5′-AAG-CAG-GGC-AGA-CAT-TGC-GAT-3′, and reverse, 5′- CAG-GAT-GTG-GCT-CTA-GGC-TAT-GT-3′; Mm Hprt1 forward, 5′-CTG-GTT-AAG-CAG-TAC-AGC-CCC-AA-3′, and reverse, 5′-CAG-GAG-GTC-CTT-TTC-ACC-AGC-3′; Mm Id1 forward, 5′- ACC-CTG-AAC-GGC-GAG-ATC-A-3′, and reverse, 5′- TCG-TCG-GCT-GGA-ACA-CAT-G-3′; Mm Socs3 forward, 5′- TTA-AAT-GCC-CTC-TGT-CCC-AGG-3′, and reverse, 5′- TGT-TTG-GCT-CCT-TGT-GTG-CC-3′; Mm Crp forward, 5′-GCT-ACT-CTG-GTG-CCT-TCT-GAT-CA-3′, and reverse, 5′- GGC-TTC-TTT-GAC-TCT-GCT-TCC-A-3′.
Immunoblots
Total protein extracts were prepared incubating cells or mouse livers in lysis buffer (200 mM Tris-HCl at pH 8, 100 mM NaCl, 1 mM EDTA, 0.5% NP-40, 10% glycerol, 1 mM sodium fluoride, 1 mM sodium orthovanadate; Complete Protease Inhibitor Cocktail; Roche). Protein content was determined by colorimetric assay (bicinchoninic acid assay, Pierce), and 50 μg of total protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to HYBOND-P membrane (GE). Membranes were probed with antiphospho-STAT3, antiphospho-SMAD1/5/8, SMAD1 (Cell Signaling), and Actin (Sigma-Aldrich) antibodies and then developed with advance enhanced chemiluminescence kit (Amersham) and visualized with the Kodak Image Station 440CF (Kodak).
Animals
C57BL/J6 (Harlan Laboratories) or BALB/c female mice (Lombardy and Emilia Romagna Experimental Zootechnic Institute) were kept on a standard diet until 8 or 9 weeks. The study was approved by the Institutional Animal Care and Use Committee of the University of Brescia, Italy. Four mice per experimental group were treated subcutaneously (SC) with saline or different doses of RO-heparins. Six hours after the last treatment, blood was collected from the tail for serum hepcidin and serum iron evaluation, and then the mice were euthanized. Liver and spleen were analyzed for messenger RNA (mRNA), protein, and/or iron content. In other experiments, mice were treated intraperitoneally (IP) with LPS (1 g/kg) with or without SC injections of RO-heparins, euthanized after 6 hours, and analyzed as described earlier. In chronic treatment experiments, mice were treated daily for 7 days with SC injections of heparins and then euthanized 6 hours after the last treatment. Mouse serum hepcidin was quantified using surface enhanced laser desorption ionization–time of flight mass spectrometry, as previously described.47-49 Spleen iron content was determined spectrophotometrically as in Roetto et al,50 with minor modifications. Serum iron was determined spectrophotometrically with a commercial kit, according to the manufacturer’s instruction (Randox Laboratories).
Treatment with heat-killed Brucella abortus
We followed the protocol recently described that causes a transient inflammatory anemia in mice.51,52 Nine-week-old female C57BL/J6 mice were treated with heat-killed Brucella abortus (HKBA), the antigen for brucellosis (Lombardy and Emilia Romagna Experimental Zootechnic Institute). The preparation of HKBA (10 mL) was precipitated, washed, and resuspended in 8 mL PBS. Animals were injected IP with 200 μL of this preparation that corresponded to 5 × 108 particles per mouse. Blood was collected from the tail, and hemoglobin (Hb) was measured on Beckman Coulter LH280 Analyzer equipment. The mice were injected subcutaneously with 100 μL of the heparins or with saline. Six hours before sacrificing, the mice were injected with another dose of heparin or saline, and liver and serum were collected for analysis.
Bmp6−/− mice
Female Bmp6−/− and wild-type mice on the CD1 background were kept on a standard diet until 9 weeks of age and were cared for in accordance with the European Convention for the Protection of Laboratory Animals. Four mice per experimental group were treated SC with a single dose of saline or RO-82 (120 mg/Kg). Livers were harvested 6 hours later, and levels of hepcidin and Id1 mRNA were assessed by quantitative PCR.26 In other experiments, mice were treated with heparins after 2 hours with IP injection of LPS (1 mg/kg) and then euthanized after 4 hours.
Statistical analysis
Data are presented as mean ± standard error of mean (SD). Data of in vitro experiments are expressed as percentage or fold increase with respect to nonstimulated cells. The data of in vivo experiments are expressed as percentage or fold increase with respect to untreated animals and represented with box plots (for a better visualization of mice distribution in each group). Comparison of values between untreated and treated cells or mice was performed by 2-tailed Student t test for unpaired data. Differences were defined as significant for P < .05 or < .001. All data are analyzed with STATGRAPHICS Centurion XV software (University of Brescia).
Results
In vitro studies
BMP6-dependent Hamp promoter activation is inhibited by gs-heparins.
The heparins analyzed in this study are listed in Table 1. RO-82, RO-68, NAc-91, and NAcRO-00 preparations were treated chemically to abolish antithrombin binding and anticoagulant activity. RO-82, RO-68, and NAcRO-00 were treated with periodate to produce gs-heparins, and NAc-91 and NAcRO-00 were N-acetylated. Their activity on the hepcidin promoter was initially analyzed in HepG2 cells transfected with HAMP promoter-luciferase construct (pGL2Luc-HAMP) and stimulated with 10 ng/mL BMP6. The assay showed that all the heparins inhibited the hepcidin promoter down to 50% of the initial signal and that RO-82 and RO-68 were more potent than NAc-91 and NAcRO-00 (supplemental Figure 1A-B, available on the Blood Web site). The finding stimulated further studies and suggested that the anticoagulant activity of heparins is not necessary for the suppression of hepcidin expression.
Gs-heparins inhibit hepcidin expression in HepG2 cells at low concentrations.
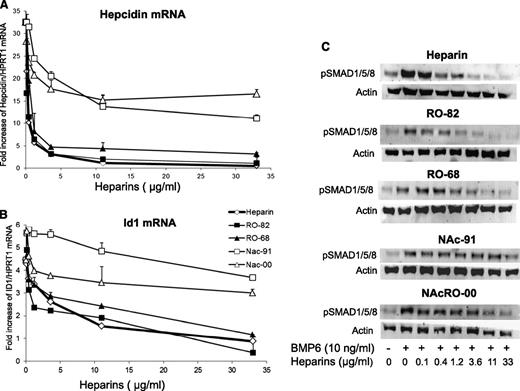
To investigate the effect of the heparins on basal or BMP6-induced hepcidin expression, we grew HepG2 cells for 16 hours, with various concentrations of the heparins in the presence or the absence of 10 ng/mL BMP6, and we analyzed hepcidin and Id1 mRNA by qRT-PCR and SMAD1/5/8 phosphorylation (pSMAD1/5/8) by western blotting. All heparins inhibited hepcidin expression both in the presence (Figure 1A) and in the absence of BMP6 (data not shown). In particular, RO-68 and RO-82 were as potent as the commercial heparin, suppressing hepcidin mRNA at concentrations greater than 1.2 μg/mL. The acetylated NAc-91 and NAcRO-00 heparins were less effective and inhibited hepcidin down to only 50% of the initial level. They suppressed also Id1 with the same order of potency, although Nac-00 was much less inhibitory than Nac-91 (Figure 1B). Immunoblot analysis confirmed that BMP6 caused an evident induction of pSMAD1/5/8, and this was strongly reduced by RO-82 and RO-68 at concentrations greater than 1.2 μg/mL (Figure 1C) and, to a lesser extent, by NAc-91 and NAcRO-00 (Figure 1C) without changing SMAD1/5/8 protein levels (data not shown). Similar experiments with a shorter, 6-hour incubation showed a similar trend (data not shown). The effect of the potent RO-68 heparin also was investigated in primary cultured mouse hepatocytes. RO-68 reduced hepcidin expression to less than 50% of the basal level at the highest concentration used (11 μg/mL; P < .05) (supplemental Figure 2). We concluded that RO-82 and RO-68 strongly inhibit hepcidin expression in human hepatic cell lines and in primary murine hepatocytes.
Glycol-split heparins reduce hepcidin and Id1 mRNA in a dose-dependent manner. (A) HepG2 cells were treated for 16 hours with nonanticoagulant heparins RO-82, RO-68, NAc-91, and NAcRO-00 at different concentrations (0.12-0.4-1.2-3.6-11-33 μg/mL) and in the presence of 10 ng/ml BMP6 . Hepcidin (A) and Id1 mRNA levels (B) were quantified with qRT-PCR in a relationship to Hprt1 mRNA. Data are means and SD of 3 different experiments and are expressed as fold increase. Anticoagulant heparin was used as control. (C) Western blotting analysis of phosphorylated SMAD1/5/8 and of Actin (as calibrator of the cell extracts) after the incubation with BMP6 and heparins. The image is representative of 3 independent experiments.
Glycol-split heparins reduce hepcidin and Id1 mRNA in a dose-dependent manner. (A) HepG2 cells were treated for 16 hours with nonanticoagulant heparins RO-82, RO-68, NAc-91, and NAcRO-00 at different concentrations (0.12-0.4-1.2-3.6-11-33 μg/mL) and in the presence of 10 ng/ml BMP6 . Hepcidin (A) and Id1 mRNA levels (B) were quantified with qRT-PCR in a relationship to Hprt1 mRNA. Data are means and SD of 3 different experiments and are expressed as fold increase. Anticoagulant heparin was used as control. (C) Western blotting analysis of phosphorylated SMAD1/5/8 and of Actin (as calibrator of the cell extracts) after the incubation with BMP6 and heparins. The image is representative of 3 independent experiments.
Gs-heparins inhibit hepcidin expression in HepG2 cells after 4 to 6 hours.
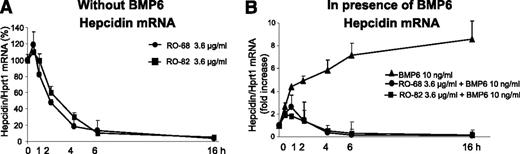
We analyzed the time course of hepcidin inhibition in HepG2 cells using heparins at the concentration of 3.6 μg/mL. Both RO-82 and RO-68 caused a minor transient increase of hepcidin mRNA at 30 minutes, followed by a full suppression of hepcidin expression after 4 hours that persisted up to 16 hours (Figure 2A). When the cells were treated with heparins in combination with 10 ng/mL BMP6, the transient initial stimulation was even stronger and persisted longer, but after 4 to 6 hours, hepcidin mRNA was fully suppressed (Figure 2B), as it occurred in the absence of BMP6. Thus, heparin action in HepG2 cells is fast and persists for a long time (up to 16 hours). This is important for in vivo kinetic studies.
Glycol-split heparins reduce basal and BMP6-induced hepcidin in HepG2 in 4 to 6 hours. (A) HepG2 cells were treated with 3.6 μg/mL glycol-split heparins RO-82 and RO-68 at different times (30 minutes-1 hour-2 hour-4 hour-6 hour-16 hour) without BMP6. The values are expressed as percentage of untreated cells (100%). (B) In presence of BMP6 (10 ng/mL). Hepcidin mRNA levels were quantified with qRT-PCR in relationship to Hprt1 mRNA. The values are expressed as fold increase of untreated cells. Data means and SD of 3 different experiments.
Glycol-split heparins reduce basal and BMP6-induced hepcidin in HepG2 in 4 to 6 hours. (A) HepG2 cells were treated with 3.6 μg/mL glycol-split heparins RO-82 and RO-68 at different times (30 minutes-1 hour-2 hour-4 hour-6 hour-16 hour) without BMP6. The values are expressed as percentage of untreated cells (100%). (B) In presence of BMP6 (10 ng/mL). Hepcidin mRNA levels were quantified with qRT-PCR in relationship to Hprt1 mRNA. The values are expressed as fold increase of untreated cells. Data means and SD of 3 different experiments.
Gs-heparins reduce hepcidin induction by inflammatory stimuli in HepG2 cells.
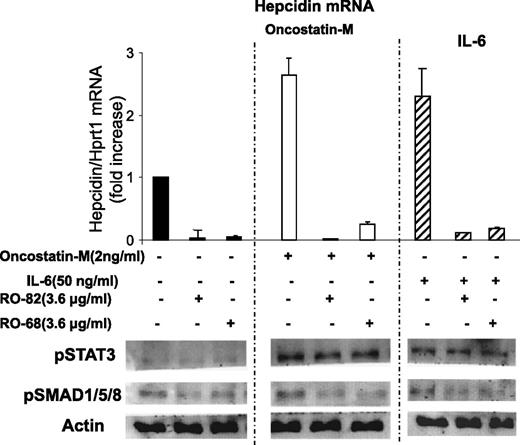
To mimic inflammatory response in vitro, the HepG2 cells were incubated with IL-6 or Oncostatin-M, which are inflammatory cytokines known to induce hepcidin expression primarily by activating STAT3. HepG2 cells were treated with 3.6 μg/mL RO-82 and RO-68 and 2 ng/mL Oncostatin-M or 50 ng/mL IL-6 for 6 hours, and hepcidin mRNA, pSMAD1/5/8, and pSTAT3 were analyzed. Oncostatin-M and IL-6 induced the phosphorylation of STAT3, but not that of SMAD1/5/8, compared with untreated cells, and caused a threefold increase in hepcidin expression (Figure 3), as expected.22,24 The presence of RO-82 and RO-68 strongly inhibited hepcidin expression and pSMAD1/5/8 without modifying pSTAT3 (Figure 3). These results confirm that heparins do not interfere with cytokine interaction with their receptors and with STAT3 signaling activation but, instead, act preferentially on the BMP/SMAD pathway.28
Glycol-split heparins reduce Oncostatin-M and IL-6-induced hepcidin mRNA and pSMAD but not pSTAT3. (Top) HepG2 cells were treated for 6 hours with 3.6 μg/mL glycol-split heparins RO-82 and RO-68 alone (solid histograms) or in combination with 2 ng/mL Oncostatin-M (empty histograms) and 50 ng/mL IL-6 (striped histograms). Hepcidin mRNA levels were quantified with qRT-PCR in relationship to Hprt1 mRNA. The values are presented as means and SD of 3 different experiments and are expressed as fold of increase of untreated cells. (Bottom) Western blotting analysis of pSMAD1/5/8, pSTAT3, and Actin. Actin was used as a calibrator of the cell extracts. The image is representative of 3 different experiments.
Glycol-split heparins reduce Oncostatin-M and IL-6-induced hepcidin mRNA and pSMAD but not pSTAT3. (Top) HepG2 cells were treated for 6 hours with 3.6 μg/mL glycol-split heparins RO-82 and RO-68 alone (solid histograms) or in combination with 2 ng/mL Oncostatin-M (empty histograms) and 50 ng/mL IL-6 (striped histograms). Hepcidin mRNA levels were quantified with qRT-PCR in relationship to Hprt1 mRNA. The values are presented as means and SD of 3 different experiments and are expressed as fold of increase of untreated cells. (Bottom) Western blotting analysis of pSMAD1/5/8, pSTAT3, and Actin. Actin was used as a calibrator of the cell extracts. The image is representative of 3 different experiments.
Chondroitin A and dermatan sulfate do not inhibit hepcidin expression.
We analyzed the effect of chondroitin A and dermatan sulfate, GAGs that differ from heparin in sugar types and lower levels of sulfation and that do not have anticoagulant activity.53 In the concentration range of 0.12 to 11 μg/mL, they did not inhibit hepcidin in HepG2 cells, both in the presence or absence of BMP6 (supplemental Figure 3).
In vivo studies
Gs-heparins reduce hepcidin in mice after 6 hours.
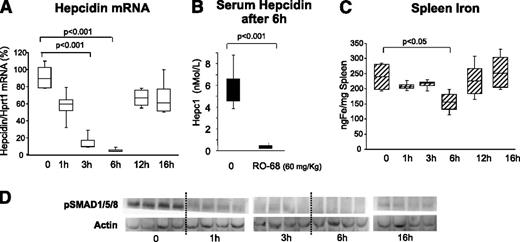
The obvious advantage of nonanticoagulant heparins is that they can be used in vivo at high concentrations and for a long time without effect on coagulation. In initial experiments, 8- to 9-week-old female BALB/c mice were treated with a single SC injection of the heparin RO-68 at 60 mg/kg and then euthanized at different times (1-16 hours). We did not observe evident effects on coagulation or hemorrhage at each of the points evaluated. The level of liver hepcidin transcript decreased to reach a minimum at 6 hours and then returned to baseline at 16 hours (Figure 4A). The level of liver phospho-SMADs decreased after 1 hour and remained low for up to 6 hours, increasing only at 16 hours (Figure 4D). Of interest was that spleen iron decreased significantly at 6 hours and then returned to baseline (Figure 4C), with a similar trend to that of hepcidin mRNA and pSMAD. To evaluate the effect of heparins on serum hepcidin, blood was drawn from 3 mice 3 weeks before the experiment and then 6 hours after the injection of heparin, immediately before death. In the control mice, we did not observe significant modifications at the 2 points, whereas in the heparin-treated animals the decrease was dramatic and serum hepcidin dropped below the detection limit of the method (Figure 4B). We concluded that the gs-heparins are effective in inducing a fast reduction of liver and serum hepcidin with transient effects on iron homeostasis.
Single dose of RO-68 (60 mg/kg) strongly inhibited liver hepcidin and spleen iron in 6 hours. Mice (8-9 weeks old) were treated SC with RO-68 (60 mg/kg) in a single dose and euthanized after 1-3-6-12-16 hours. (A) Hepcidin mRNA was quantified by qRT-PCR in relationship to Hprt1 mRNA. (B) Serum hepcidin was measured by surface enhanced laser desorption ionization–time of flight mass spectrometry after 6 hours of treatment. (C) Spleen iron was measured spectrophotometrically. (D) pSMAD1, pSMAD15, and pSMAD18 were evaluated by western blotting analysis. Actin was used as calibrator. Data are representative of 4 mice/point and are expressed with box plot histograms. Hepcidin level is expressed as percentage of untreated mice (100%)
Single dose of RO-68 (60 mg/kg) strongly inhibited liver hepcidin and spleen iron in 6 hours. Mice (8-9 weeks old) were treated SC with RO-68 (60 mg/kg) in a single dose and euthanized after 1-3-6-12-16 hours. (A) Hepcidin mRNA was quantified by qRT-PCR in relationship to Hprt1 mRNA. (B) Serum hepcidin was measured by surface enhanced laser desorption ionization–time of flight mass spectrometry after 6 hours of treatment. (C) Spleen iron was measured spectrophotometrically. (D) pSMAD1, pSMAD15, and pSMAD18 were evaluated by western blotting analysis. Actin was used as calibrator. Data are representative of 4 mice/point and are expressed with box plot histograms. Hepcidin level is expressed as percentage of untreated mice (100%)
Gs-heparins reduce chemically induced inflammation in mice.
To induce an inflammatory response, female BALB/c mice were treated with an IP injection of LPS (1 g/kg), together with a SC injection of saline or of RO-82 and RO-68 (at 20 mg/kg and 120 mg/kg); the mice were euthanized 6 hours later. We did not observe evident effects on coagulation or hemorrhage in each experimental groups. LPS induced a 3- to 4-fold increase of liver hepcidin mRNA (Figure 5A) and a significant decrease in serum iron (Figure 5E), as expected. Both heparins at the dose of 20 mg/kg reduced the effect of LPS stimulus on hepcidin mRNA (twofold) and did not affect serum iron. At a higher dose (120 mg/kg), they abolished the LPS effect both on hepcidin mRNA, which decreased below basal level, and on serum iron (Figure 5A,E). Id1 expression was not affected by LPS but decreased significantly after the treatments with high doses of heparins (Figure 5B). To monitor inflammatory status, we analyzed the level of liver Socs3 and of Crp mRNAs. LPS treatment caused a significant increase of Socs3 (30-fold) and Crp (twofold) levels. Their levels did not change after administration of RO-68 but decreased with the high dose of RO-82 heparin (Figure 5C-D). These results indicate that gs-heparins also reduce hepcidin expression in mice with chemically induced inflammation, that they act preferentially on SMAD signaling, and that even at the high dose of 120 mg/kg, no effect on coagulation was observed.
A single treatment of RO heparins (20 or 120 mg/kg) abolished LPS-dependent induction of hepcidin in mice. Mice (8-9 weeks old) were treated with LPS (1 g/kg) with or without a single dose of RO-heparins (20 or 120 mg/kg of RO-82 or RO-68) and euthanized after 6 hours. qRT-PCR was used for mRNA quantification of (A) hepcidin, (B) Id1, (C) Socs3, and (D) Crp in relationship to Hprt1 mRNA. The values are presented with box plot histograms and are expressed as fold increase (1) or as percentage (100%) of untreated mice. (E) Serum iron was measured spectrophotometrically.
A single treatment of RO heparins (20 or 120 mg/kg) abolished LPS-dependent induction of hepcidin in mice. Mice (8-9 weeks old) were treated with LPS (1 g/kg) with or without a single dose of RO-heparins (20 or 120 mg/kg of RO-82 or RO-68) and euthanized after 6 hours. qRT-PCR was used for mRNA quantification of (A) hepcidin, (B) Id1, (C) Socs3, and (D) Crp in relationship to Hprt1 mRNA. The values are presented with box plot histograms and are expressed as fold increase (1) or as percentage (100%) of untreated mice. (E) Serum iron was measured spectrophotometrically.
Chronic treatments with gs-heparins reduce hepcidin expression and spleen iron.
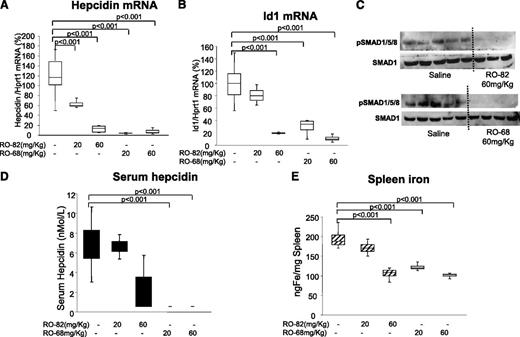
To evaluate the effect of the gs-heparins on body iron homeostasis, C57BL/J6 mice were treated daily for 7 days with saline or with RO-82 and RO-68 at a concentration of 20 to 60 mg/kg per day. We did not observe effects on coagulation. RO-68 seemed to be the most effective in suppressing liver mRNA and serum hepcidin at both doses (Figure 6A,D). RO-68 also suppressed Id1 mRNA and reduced spleen iron (Figure 6B,E). RO-82 was apparently less potent, and at the concentration of 20 mg/kg per day, halved liver hepcidin mRNA, whereas serum hepcidin, spleen iron, and Id1 mRNA did not change significantly. At the higher dose of 60 mg/kg per day, it fully suppressed hepcidin and SMAD1/5/8 phosphorylation (Figure 6A,C). We concluded that gs-heparins may be used in chronic treatment to induce iron mobilization (Figure 6E).
Chronic treatment with RO-82 and RO-68 (20-60 mg/kg daily for 1 week) inhibited liver hepcidin and spleen iron. Mice (8-9 weeks old) were treated with RO-82 and RO-68 (20-60 mg/kg daily for 1 week) and euthanized. Hepcidin (A) and Id1(B) mRNA were quantified by qRT-PCR in relationship to Hprt1 mRNA. Data are representative of 4 mice/point and are expressed with box plot histograms. Hepcidin and Id1 level are expressed as percentage of untreated mice (100%). pSMAD1/5/8 and SMAD1 were evaluated in untreated and treated (60 mg/kg RO-82 and RO-68) mice by western blotting (C). Serum hepcidin was measured by surface enhanced laser desorption ionization–time of flight mass spectrometry (D), and spleen iron was measured spectrophotometrically (E).
Chronic treatment with RO-82 and RO-68 (20-60 mg/kg daily for 1 week) inhibited liver hepcidin and spleen iron. Mice (8-9 weeks old) were treated with RO-82 and RO-68 (20-60 mg/kg daily for 1 week) and euthanized. Hepcidin (A) and Id1(B) mRNA were quantified by qRT-PCR in relationship to Hprt1 mRNA. Data are representative of 4 mice/point and are expressed with box plot histograms. Hepcidin and Id1 level are expressed as percentage of untreated mice (100%). pSMAD1/5/8 and SMAD1 were evaluated in untreated and treated (60 mg/kg RO-82 and RO-68) mice by western blotting (C). Serum hepcidin was measured by surface enhanced laser desorption ionization–time of flight mass spectrometry (D), and spleen iron was measured spectrophotometrically (E).
Gs-heparins facilitate recovery after HKBA induced anemia.
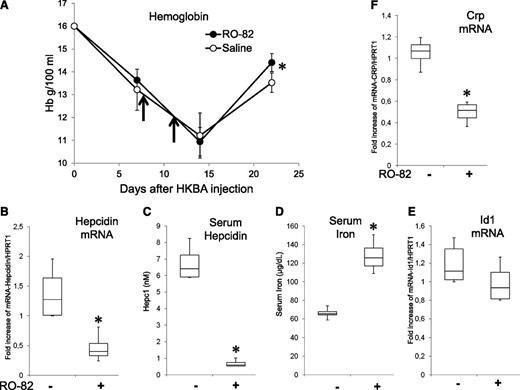
Single injections of HKBA were shown to induce an inflammatory anemia that lasted more than 2 weeks, a good mouse model of inflammatory anemia.51,52 We confirmed that after a single injection of the HKBA antigen at a concentration equivalent to 5 × 108 particles per mouse (females, 9 weeks old) we produced a decrease in Hb level from 16 to about 11 g/dL after 2 weeks. Treatment with 2 SC injections of 120 mg/kg R0-82 heparin at day 8 and day 11 had no effect on the decline of Hb but improved the recovery at day 22, when the level of Hb in the RO-82 treated mice was significantly higher than that of the saline controls (Figure 7A). Mice were injected again with RO-82 (120 mg/kg) and sacrificed after 6 hours. In the RO-82-treated mice, the mRNAs of liver hepcidin and Crp were significantly decreased, and serum hepcidin was strongly downregulated, whereas serum iron was significantly increased (Figure 7B-D,F). Id1 mRNA decreased, but nonsignificantly (Figure 7E).
Treatment with RO-82 (120 mg/kg) facilitates the recovery after HKBA-induced anemia. (A) Female (9-week-old) mice were treated with a single IP injection of HKBA (∼5 × 108 particles/mouse) at day 0. Then they were treated on day 8 and 11 (vertical arrows) with a dose of RO-82 heparin (120 mg/kg) or with saline, and the Hb level was monitored at days 7, 14, and 22. After the last bleeding, the mice were treated with another injection of RO-82 or saline, euthanized after 6 hours, and had their liver and serum analyzed. Transcripts of liver hepcidin, Id1, and Crp were evaluated by qRT-PCR, referred to Hprt1, and expressed as fold increase (B,E-F). The concentration of serum hepcidin (C) and serum iron (D) are also shown. At least 3 animals per group asterisks (*) indicate a significant difference (P < .05).
Treatment with RO-82 (120 mg/kg) facilitates the recovery after HKBA-induced anemia. (A) Female (9-week-old) mice were treated with a single IP injection of HKBA (∼5 × 108 particles/mouse) at day 0. Then they were treated on day 8 and 11 (vertical arrows) with a dose of RO-82 heparin (120 mg/kg) or with saline, and the Hb level was monitored at days 7, 14, and 22. After the last bleeding, the mice were treated with another injection of RO-82 or saline, euthanized after 6 hours, and had their liver and serum analyzed. Transcripts of liver hepcidin, Id1, and Crp were evaluated by qRT-PCR, referred to Hprt1, and expressed as fold increase (B,E-F). The concentration of serum hepcidin (C) and serum iron (D) are also shown. At least 3 animals per group asterisks (*) indicate a significant difference (P < .05).
Gs-heparins also reduce hepcidin in Bmp6−/− mice.
In vitro data indicate that heparins inhibit hepcidin expression mainly by sequestering BMP6 and interfering with its signaling.28 Thus, it was of interest to verify whether heparins showed the same effect in Bmp6−/− mice. The males of this strain have very low expression of hepcidin in the liver; therefore, we used females that have a residual level of hepcidin mRNA (about 20% of that of wild-type mice of the same background), probably induced by other ligands of the BMP subfamily. Bmp6−/− females and wild-type controls (Bmp6+/+) were treated with a single SC injection of 120 mg/kg RO-82 and sacrificed after 6 hours. In the Bmp+/+ mice, the level of hepcidin mRNA dropped to about 10% of the basal, and, more interestingly, also in the Bmp6−/− mice, there was an evident decrease in hepcidin mRNA after the treatment (supplemental Figure 4A). A similar trend was observed for Id1 mRNA, although the differences were significant only in the wild-type mice (supplemental Figure 4B). We also checked whether heparin could limit hepcidin induction by LPS in these mice. Whereas, as shown previously, LPS increased hepcidin mRNA 3- to 4-fold in Bmp6−/− females, this induction was entirely suppressed by RO-82 heparin (supplemental Figure 4C). A similar trend was observed for Id1 mRNA, with an even higher inhibition by heparin in the LPS-treated mice (supplemental Figure 4D).
Discussion
We have previously demonstrated that heparin inhibits hepcidin expression in hepatoma HepG2 cells and in mice, even at low pharmacological concentrations.28 However, the use of commercial anticoagulant heparins in vivo is difficult, as they can be used only at low doses and for a short time to limit bleeding complications. This problem does not occur with heparins with low/null anticoagulant activities. Such heparins have been produced, and they have various biological activities, perhaps related to the heparan sulfates, ubiquitous on cell surface, which do not have anticoagulant activity. Most anticoagulant activity is a result of the high-affinity binding to antithrombin of a pentasaccharide that contains an unusually fully sulfated glucosamine flanked by glucuronic acid (GlcA) in a peculiar sequence named ATBR.54 This structure can be altered by chemical reactions. A well-characterized one is the oxidation with meta-periodate that breaks the bond between 2 hydroxyl groups present on nonsubstituted uronic acids (glycol-split). This alters the essential GlcA residue within the ATBR and drastically reduces the anticoagulant activity of heparin.45 A similar effect is obtained by N-acetylation, which modifies the essential fully sulfated glucosamine of the ATBR sequence. The nonanticoagulant heparins produced in these ways retain biological activities unrelated to coagulation, such as antiheparanase,55 anti-inflammatory43 and antiangiogenesis activity.42,56 Some of them have been proposed for clinical treatment of solid tumors.57 They have been used in mice without adverse effects, even at doses as high as 120 mg/kg per day for 28 days.41 In this work, we used 3 glycol-split heparins and a single N-acetylated heparin to verify whether they retain antihepcidin activity as the unfractionated compounds. All the gs-heparins had antihepcidin activity in HepG2 cells, but the 2 N-acetylated (NAc-91 and NAcRO-00) heparins were less potent than the other ones (RO-82 and RO-68), which behaved similarly to the commercial unfractionated heparins. RO-68 also was effective in primary hepatocytes. In HepG2 cells, RO-82 and RO-68 fully inhibited hepcidin mRNA at the pharmacological concentration of 1 to 4 μg/mL. The suppression occurred in 4 hours, both in the presence and absence of BMP6 induction, and lasted for up to 16 hours. Interestingly, the inhibition also was evident in the presence of the inflammatory cytokines Oncostatin-M and IL-6, which activate the STAT3 signaling but not the BMP/SMAD pathway. Altogether, the studies in HepG2 cells did not show major differences among unfractionated heparin, RO-82, and RO-68, which have similar molecular weights and slightly different sulfation degree (RO-68 is 2O desulfated). However, N-acetylation of glucosamine strongly reduced antihepcidin activity, both before and after glycol splitting. This suggests that a high density of negative charges is important for this activity and that the introduction of a flexible residue in place of sulfate residues does not impair the interaction.
More interesting is the in vivo use of the gs-heparins, as their low/absent anticoagulant activity allows the use of doses higher than those of commercial heparins. In fact, the subcutaneous injections of heparins up to 120 mg/kg (about 2.5 mg/mouse) did not cause bleeding or other adverse effects in the mice, according to previous reports.41 The heparins RO-82 and RO-68 inhibited liver hepcidin mRNA by 90% of the basal value after 3 to 6 hours; that is, in the same time range observed in HepG2 cells. However, the recovery in vivo was faster, and after 12 hours, the level returned close to the basal one. This suggests that an optimized treatment may consist in injections at 6-hour intervals. Repeated injections for 1 week were effective, although not yet optimized. Relatively low doses of RO heparins, particularly RO-68, fully inhibited hepcidin mRNA and serum protein, as well as reduced spleen iron concentration, an important index of iron mobilization from macrophages. The modified heparins also were tested under conditions of LPS-induced experimental inflammation.. We found that the gs-heparins reduced/abolished the effects of LPS treatment on iron and hepcidin homeostasis. This is illustrated by the near total inhibition of the changes of hepcidin and serum iron levels induced by a single injections of LPS observed with the coadministration of RO-heparins at 120 mg/kg, particularly of RO-68. This effect is not a result of a sequestering of LPS by the heparin, as the inflammatory pathways via STAT3 (Socs3 mRNA) and nuclear factor kappa-light-chain-enhancer of activated B cells (Crp mRNA) were not affected by the treatments. Thus, in vivo as well in vitro, the RO-heparins seem to inhibit preferentially the BMP/SMAD pathway, which induces hepcidin and Id1 mRNAs. We conclude that heparins behave similarly to soluble HJV.fc that targets specifically the BMP/HJV/SMAD pathway and inhibits both iron- and IL-6-induced hepcidin expression in mice.58
We also used HKBA-treated mice as a model of inflammatory anemia that was previously used to evaluate the efficiency of antihepcidin antibody.52 After a single HKBA injection, the Hb level dropped to about 11 g/dL in 2 weeks; after this period, it started recovering. Two injections with high doses of RO-82 heparin (120 mg/kg) had no effect on Hb decline in the 2 weeks after HKBA treatment. However, high doses of antihepcidin antibodies also did not improve anemia in this model in the absence of a cotreatment with erythropoiesis-stimulating agents.51,52 Of interest, RO-82 heparin not only improved the recovery of anemia at day 22 but also significantly reduced serum hepcidin, the mRNAs of hepcidin and Crp, and increased serum iron to physiological levels. Thus, some positive effects of the RO-82 heparin on a model with inflammatory anemia were observed, and they will probably improve after optimization of the treatments. This will involve the selection of the most effective heparin. The less-sulfated RO-68 seemed to be more potent in untreated mice (Figure 5), whereas the more-sulfated RO-82 displayed a slightly higher antihepcidin activity in LPS-treated mice, perhaps because it is more effective in reducing the inflammatory markers Socs3 and Crp (Figures 4 and 7). In fact, it has been already observed that a higher level of sulfation as in RO-82 is associated with a higher-affinity to heparin-binding molecules, such as heparanase,44 and possibly also those involved in inflammatory responses.
Because the in vitro data indicate that heparins act preferentially by inhibiting BMP6 activity,28 it was of interest to verify whether they can still suppress hepcidin in Bmp6−/− mice. Only females could be used, as the males have too low/absent hepcidin expression.19 Interestingly, heparin reduced the residual level of hepcidin in these mice, which was paralleled by the inhibition of Id1, an index of activation of the BMP/SMAD pathway. This is in agreement with previous findings that the expression of hepcidin in female Bmp6−/− mice is linked to residual activity of the BMP/SMAD pathway stimulated by factors other than BMP6. Collectively, the in vitro and in vivo data so far obtained strongly indicate that the heparins suppress hepcidin expression by targeting the BMP/SMAD signaling. This involves the binding of BMPs, mainly BMP6 and, in its absence, other BMPs.
In conclusion, our data show that glycol-split nonanticoagulant heparins are effective and convenient molecules to inhibit hepcidin in vitro and in vivo in 3 different strains of mice under conditions of inflammation, and even in the absence of BMP6. The optimized structure of heparin for hepcidin inhibition has yet to be identified, but present data suggest it may not be very different from the nonanticoagulant heparins now used in clinical studies that have shown little or no toxicity (eg, ClinicalTrials.gov, NCT01764880). Nonanticoagulant heparins have antihepcidin, anti-inflammatory, and also antitumor activity, and they may be useful agents for the treatment of inflammatory anemia in cancer and chronic diseases.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Marie-Paule Roth for helpful discussion and reading the manuscript, Dr Clara Camaschella and Dr Laura Silvestri for providing the plasmids pGL2Luc-HAMP and pGL2TK-RL, and Dr E. Ascari from the laboratory of Spedali Civili of Brescia for Hb determination.
This work was partially supported by Fondazione Cariplo Grant 2012-0570 (P.A. and A.N.), by Grant MIUR-PRIN-11 (P.A.), and by Italian Ministry of University and Research Grant. 200989KXFN (D.G.).
Authorship
Contribution: M.P. designed and performed the research, analyzed data, and wrote the paper; M.A. performed research, analyzed data, and contributed to the preparation of the paper; A.N. produced the modified nonanticoagulant heparins and contributed to the preparation of the paper; N.C. performed the research on hepcidin dosage and contributed to the preparation of the paper; D.G. contributed to the preparation of the paper; M.C. contributed in the isolation of primary hepatocytes; M.B. produced the modified nonanticoagulant heparins; F.M. contributed to the experiments on mice; D.F. contributed to the preparation of the paper; H.C. designed the experiments on Bmp6−/− mice and analyzed the data; C.B.-F. designed and performed the experiments on Bmp6−/− mice; and P.A. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paolo Arosio Department MMT, University of Brescia, Viale Europa 11, 25123 Brescia, Italy; e-mail: arosio@med.unibs.it.