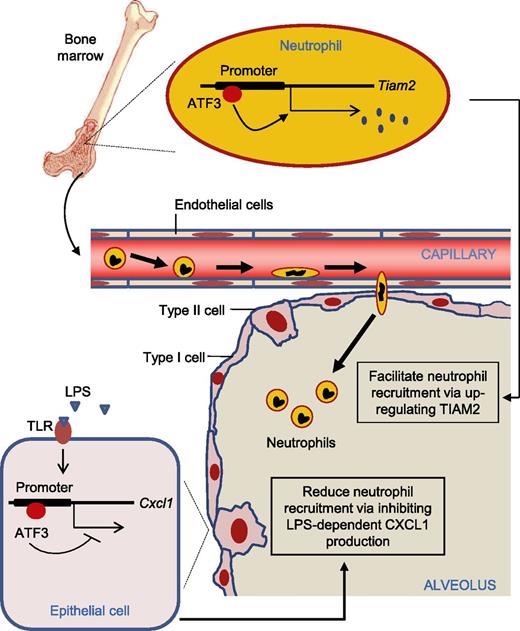
In this issue of Blood, Boespflug et al report that activating transcription factor 3 (ATF3), a member of the ATF/cyclic AMP response element-binding (ATF/CREB) family of transcription factors, plays a crucial role in regulating neutrophil recruitment during lung inflammation.1
ATF3 inhibits LPS-dependent CXCL1 production by lung epithelia, which would tend to reduce neutrophil recruitment. On the other hand, neutrophils require ATF3 expression for normal and accurate migration, most likely through developmental regulation of TIAM2 expression, which would tend to facilitate neutrophil recruitment.
ATF3 inhibits LPS-dependent CXCL1 production by lung epithelia, which would tend to reduce neutrophil recruitment. On the other hand, neutrophils require ATF3 expression for normal and accurate migration, most likely through developmental regulation of TIAM2 expression, which would tend to facilitate neutrophil recruitment.
ATF3 is a member of the ATF/CREB family of basic leucine zipper (bZIP) transcription factors.2,3 Basal ATF3 expression is low in most cell types but is rapidly and transiently elevated in response to various stimuli, including those leading to Toll-like receptor (TLR) activation. ATF3 is generally regarded as a negative regulator of immune function, functioning as part of a negative feedback loop to suppress TLR-mediated cytokine expression.4-6 ATF3-deficient primary macrophages produce elevated amounts of interleukin (IL)-6 and IL-12 cytokines in response to a range of activated TLRs, and ATF3-deficient mice administered lipopolysaccharide (LPS) intraperitoneally have significantly elevated serum IL-6 and IL-12 levels compared with wild-type controls. ATF3-mediated induction and regulation of immune responses are highly cell type and stimulation dependent, and therefore understanding the immunoregulatory role(s) of ATF3 demands careful evaluation in a variety of specific immune contexts.
Boespflug and colleagues evaluate the role of ATF3 in acute lung inflammation and reveal a previously unappreciated role for ATF3 in the regulation of LPS-induced CXC chemokine ligand 1 (CXCL1) production. ATF3 deficiency resulted in increased airway CXCL1 production at low doses of LPS (<10 ng/mL), an effect that was overcome with higher LPS doses. This result is consistent with a previous report that ATF3 inhibits immune responses to transient signals, yet, following persistent TLR signaling, ATF3 silences its expression via autoinhibition.7 Using a bone marrow (BM) transplantation assay, they further demonstrate that resident lung epithelial cells are the likely locus of ATF3-mediated regulation of LPS-driven CXCL1 in the airway rather than recruited or resident BM-derived cells.
However, the most surprising result in this study was the lack of neutrophil recruitment to the lungs of ATF3-deficient mice, despite significant increases in CXCL1, a major chemokine known to recruit neutrophils during acute lung inflammation. ATF3-deficient mice showed no changes in airway neutrophil numbers following intratracheal LPS challenge, and migration of ATF3-deficient neutrophils was impaired in vitro. Because mature neutrophils do not express significant levels of ATF3, they argued that the intrinsic migratory defects of ATF3-deficient neutrophils were likely to be a function of events occurring prior to neutrophil maturation, including control of target gene expression during myeloid differentiation. By comparing global gene expression in wild-type and ATF3-deficient neutrophils, they identified Tiam2 as the gene responsible for the observed chemotaxis defect. Both human and mouse TIAM2 promoters contain consensus ATF3-binding sites. TIAM2 mRNA and protein expression increased during myeloid cell differentiation into neutrophils, yet TIAM2 protein expression was nearly completely lost from ATF3−/− neutrophils. TIAM2 knockdown in wild-type neutrophils phenocopied the migration defects of ATF3-deficient neutrophils.
Of more importance from a cell biology perspective, the investigators were able to correlate abolished TIAM2 expression levels with impaired focal adhesion (FA) disassembly and elevated F-actin polymerization, albeit with considerable uncertainty due to technical difficulties in rescuing the observed defect by overexpressing TIAM2 in ATF3-deficient neutrophils. Of note, TIAM2 is a specific Rac1-activating guanine exchange factor that controls cellular motility by promoting FA disassembly in epithelial cells; TIAM2 knockdown in epithelial cells results in reduced rates of FA disassembly and consequently enlarged FAs and reduced migratory speeds.8 Thus, TIAM2-mediated dysregulated focal complex disassembly is a plausible molecular mechanism to explain reduced ATF3-deficient neutrophil chemotaxis. In addition to increased numbers of focal complexes, they observed striking increases in F-actin polymerization in ATF3-deficient neutrophils. It will be interesting to determine whether this is secondary to dysregulated focal complex disassembly or represents an additional ATF3-dependent effect.
Neutrophils are recruited to infected tissues in response to inflammatory stimuli to protect their host. However, excessive neutrophil accumulation or hyperactivation of neutrophils can be detrimental to the system, and neutrophil recruitment and function need to be tightly regulated. The data discussed here definitively demonstrate that ATF3 has a dual role in acute lung inflammation (see figure). On the one hand, negative feedback regulation by ATF3 is critical for the prevention of acute inflammatory syndromes by limiting proinflammatory cytokine expression. On the other hand, neutrophils require ATF3 expression for normal and accurate migration. Given its dual role in neutrophil recruitment, ATF3 could be a promising therapeutic target for modulating neutrophil trafficking in various infectious and inflammatory diseases.
In conclusion, Boespflug et al establish that ATF3 and TIAM2 are keys regulators of neutrophil recruitment in acute lung inflammation. However, several important questions remain unanswered. For example, the current study focuses on TIAM2, a factor directly related to chemotaxis. As revealed in this study, ATF3-mediated effects on migration and chemotaxis are likely to be a function of events occurring prior to neutrophil maturation, and therefore, the chemotactic effects may be indirect. It will therefore be interesting to see whether other ATF3 downstream targets also induce chemotaxis defects. In addition, elucidating the contribution of ATF3 to neutrophil recruitment during infection and inflammation in physiologically relevant disease models (eg, bacterial pneumonia) is worthy of future investigation for clinicopathological correlation.
Conflict-of-interest disclosure: The author declares no competing financial interests.