In this issue of Blood, Jitschin et al provide compelling evidence that increased mitochondrial oxidative phosphorylation and production of reactive oxygen species (ROS) leads to reduced T-cell immune function.1
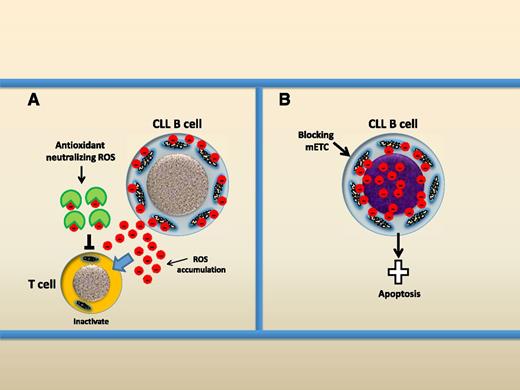
Two opposite therapeutic redox-modulating strategies for the treatment of CLL. (A) Using antioxidant to neutralize ROS, CLL cells produce and release ROS into the microenvironment and ROS are dangerous to T cells. Antioxidant can neutralize ROS and prevent ROS-mediated damage to T cells. (B) Rapid induction of ROS. Blocking the mETC causes rapid ROS production by mitochondria and this could induce apoptotic cell death of CLL cells.
Two opposite therapeutic redox-modulating strategies for the treatment of CLL. (A) Using antioxidant to neutralize ROS, CLL cells produce and release ROS into the microenvironment and ROS are dangerous to T cells. Antioxidant can neutralize ROS and prevent ROS-mediated damage to T cells. (B) Rapid induction of ROS. Blocking the mETC causes rapid ROS production by mitochondria and this could induce apoptotic cell death of CLL cells.
A half-century ago, Warburg postulated that mitochondrial respiration is impaired in cancer cells and the failure of adenosine triphosphate (ATP) production by mitochondria is compensated by an increased anaerobic glycolysis.2 Although this theory has been confirmed in many solid tumors, less is understood about the metabolism of leukemic cells. It was demonstrated that chronic lymphocytic leukemia (CLL) cells have increased mitochondrial biogenesis3 and ROS production compared with normal B cells and that high levels of ROS have been detected in more aggressive CLL cells.4 However, the source of ROS production in CLL cells and whether the oxidative stress could affect immune function of the T cell are not clear. In this issue, Jitschin et al present evidence that (CLL) cells have elevated mitochondrial oxidative phosphorylation but do not have increased aerobic glycolysis, demonstrating that circulating CLL cells and solid tumor cells rely on distinct bioenergetics.1 Importantly, the greater mitochondrial respiration leads to increased ROS production and intrinsic oxidative stress.
First, the authors determined the status of oxidative stress in the sera from 63 CLL patients compared with healthy donors. DNA-(8-OHdG) and lipid oxidation (malondialdehyde [MDA]) were used as surrogate markers for ROS. They found that CLL cells have significantly increased levels of both 8-OHdG and MDA. Higher levels of ROS correlated with numbers of circulating CLL cells. Immune cells are sensitive to ROS-induced damage or even cell death by apoptosis. Increased levels of ROS in the microenvironment can be detrimental for immune cells. Our previous study demonstrated that CD4+ and CD8+ T cells in CLL show impaired immunologic synapse formation.5 Jitschin et al detected that CD4+ and CD8+ T cells in ROShigh patients showed significantly reduced levels of CD3ζ chain expression. Particularly, ROShigh patients have less activated phenotype of CD4+ cells. The addition of the antioxidant N-acetyl-cysteine (NAC) prevented CLL cell-mediated T-cell dysfunction.
Intracellular ROS are often generated via the catalytic action of NADHP oxidase (NOX) and/or the mitochondrial electron transport chain (mETC) which is cell type-dependent. The authors found that CLL cells have lower expression of gp91, a subunit of NOX, compared with normal B cells. CLL and normal B cells showed a similar capacity in responding to stimulation of NOX by either an increase in its substrate or induction of respiratory burst by phorbol 12-myristate 13-acetate, indicating that NOX is not responsible for ROS overproduction by CLL cells. Gene expression of intracellular antioxidants catalase and heme oxygenase-1 (HO-1) was significantly increased in CLL cells. By contrast, mitochondrial manganese superoxide dismutase (MnSOD) expression was significantly reduced in CLL cells. Jitschin and colleagues suggested that significantly increased mitochondrial superoxide production might be caused, at least in part, by reduced levels of MnSOD. To further identify the ROS generation system in CLL cells, the mitochondrial targeting antioxidant MitoQ and NOX inhibitor diphenyleneiodonium were used to inhibit ROS production. Only MitoQ led to a significant reduction of ROS production, indicating that mitochondria are the source of ROS production in CLL cells.
Using multiple techniques, the authors thoroughly demonstrate that CLL cells have an increased mitochondrial biogenesis, such as increased mitochondrial mass, membrane potential, ATP production, mitochondrial DNA copy numbers, oxygen consumption, and mETC activity. Mitochondrial transcription factor (TFAM) is highly expressed in CLL cells and this may lead to an increased mitochondrial mass. However, aerobic glycolysis is not increased in CLL cells compared with normal B cells. This supports our previous finding that leukemic cells meet high energy demand by increasing mETC activity, rather than increasing aerobic glycolysis.6 The superoxide radical is generated by mitochondrial complex I and III as an incompletely reduced by-product. ROS production is often induced when mETC is uncoupled with ATP synthesis.7 Interestingly, this study found that the mitochondria in CLL cells are in a perfectly coupled condition.
To connect ROS production and mitochondrial biogenesis, Jitschin et al manipulated redox status by treating CLL cells with antioxidants NAC or MitoQ and found that expression of both TFAM and HO-1 was reduced after treatment with an antioxidant. Interestingly, inhibition of HO-1 by SnPP significantly reduced TFAM expression. Therefore, the authors drew an indirect interconnection between ROS production, cellular adaption to ROS, and mitochondrial biogenesis. Finally, PK11195, a benzodiazepine derivate inhibitor for F1F0-ATPase, the complex V of mETC, was used to block oxidative phosphorylation. PK11195-mediated overproduction of superoxide selectively induced apoptosis in CLL B cells, leaving T cells and normal B cells unaffected.
Therefore, the authors propose 2 opposite therapeutic redox-manipulating strategies for CLL (see figure). First, treatment of CLL cells using antioxidants could neutralize endogenous ROS and protect the immune system. Second, induction of ROS overproduction by targeting mETC could selectively kill CLL cells. Thus, mitochondria, the source of dangerous power in CLL, need to be controlled in favor of the immune system and can be used as a killer within the CLL cells. This study improves our understanding that leukemic cells circulating in a normoxic condition rely on mitochondrial oxidative phosphorylation for energy supply. This is distinct from solid tumor cells which grow in the hypoxia condition with dysfunctional mitochondria and therefore mainly rely on anaerobic glycolysis. Tumor cells produce higher levels of ROS by dysfunctional mitochondria. The causes of ROS overproduction by CLL mitochondria pose challenging questions. It is not yet clear how increased ROS levels are generated by functional coupled mETC and whether this is merely due to increased mitochondrial biogenesis in CLL cells.
Conflict-of-interest disclosure: The authors declare no competing financial interests.