In this issue of Blood, Jun et al, through the study of neutrophils deficient in the glucose-6-phosphate transporter, describe a novel role for the peroxisome proliferator-activated receptor-γ (PPARG) pathway in the regulation of key neutrophil functions and link this to concomitant hypoxia-inducible factor (HIF) 1α stabilization.1
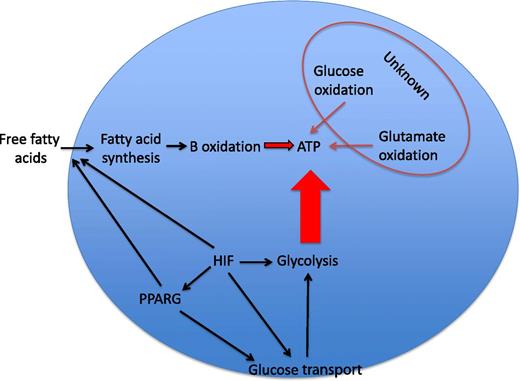
Neutrophil energetics. Glycolysis is regarded as the dominant source of ATP in neutrophils. The relative contributions of glucose oxidation, glutamate oxidation, and β-oxidation of fatty acids to ATP synthesis in the neutrophil are largely unknown and variably important in other cell types. HIF-1α is an important transcriptional regulator of glucose transporters and of the majority of the enzymes in the glycolytic pathway and is thus a major determinant of ATP levels in myeloid cells. HIF-1α has more recently been reported to regulate PPARG, which itself can increase glucose uptake and regulate lipid metabolism in a range of cell types. Substrate availability may also be critical in determining the consequences of HIF-1α or PPARG activation for the energetic and functional status of the neutrophil.
Neutrophil energetics. Glycolysis is regarded as the dominant source of ATP in neutrophils. The relative contributions of glucose oxidation, glutamate oxidation, and β-oxidation of fatty acids to ATP synthesis in the neutrophil are largely unknown and variably important in other cell types. HIF-1α is an important transcriptional regulator of glucose transporters and of the majority of the enzymes in the glycolytic pathway and is thus a major determinant of ATP levels in myeloid cells. HIF-1α has more recently been reported to regulate PPARG, which itself can increase glucose uptake and regulate lipid metabolism in a range of cell types. Substrate availability may also be critical in determining the consequences of HIF-1α or PPARG activation for the energetic and functional status of the neutrophil.
Glycogen storage disease type Ib (GSD-Ib) is a rare autosomal-recessive condition in which deficiency in the glucose-6-phosphate (G6P) transporter results in a defect in cycling of glucose and G6P between the endoplasmic reticulum and the cytoplasm. Patients with this condition characteristically display a phenotype of neutropenia and neutrophil dysfunction. In the current edition of Blood, Jun et al1 report that viable peripheral blood neutrophils (ie, following exclusion of the significant subpopulation that displays biochemical markers of apoptosis) isolated from patients with GSD-Ib display defective superoxide generation, chemotaxis, and calcium mobilization. In keeping with other previously described disorders of G6P/glucose metabolism, they describe a reduction in basal levels of G6P, lactate, adenosine triphosphate (ATP), and reduced NAD phosphate in these cells. Interestingly, many (although not all) patient samples show increased expression of the oxygen-sensitive transcription factor HIF-1α and also of a nuclear receptor and transcriptional regulator, PPARG. The authors replicated the functional defects in neutrophils from healthy donors by treatment with a PPARG agonist, rosiglitazone, and partially rescued the functional defects observed in the GSD-Ib patient neutrophils with a PPARG antagonist, GW9662.
Together, these data raise interesting questions of broad interest with regard to the regulation of myeloid cell function and the interface with metabolic flux. GSD-Ib patient neutrophils show HIF-1α upregulation in the context of defective cellular energetics. Loss of HIF-1α has previously been linked to global deficiencies in myeloid cell function, where reduced ATP availability is associated with profound functional defects2 and also with lack of survival under conditions of reduced oxygen availability.3 It is, therefore, important to be mindful that although Jun et al describe increased expression of HIF-1α in GSD-Ib neutrophils, they do not demonstrate GSD-Ib neutrophil dysfunction to be a direct consequence of HIF-1α stabilization. Attempts to replicate the functional phenotypes in healthy neutrophils with the 2ME2 compound, whose actions include loss of HIF stabilization, were potentially confounded by other described actions of this compound.4 What is clear is that key neutrophil functions are exquisitely sensitive to changes in intracellular ATP availability and that although the many hundreds of HIF target genes include those regulating cellular glycolysis, and thus ATP generation, the interactions are likely to be complex (see figure). For instance, metabolic intermediates have themselves been shown to regulate HIF stabilization, leading to the potential for disordered glucose cycling to result in disordered HIF activation5 and inappropriate activation of proinflammatory signaling pathways.6 Thus, the phenotype observed by Jun et al could equally represent a phenotype of disordered HIF stabilization as a consequence of defective glucose cycling, with the HIF-1α–mediated effects on glycolysis and glucose uptake observed in normal cells being ineffective because of the underlying cellular defect.
A further interesting finding in this paper is the upregulation of PPARG in neutrophils of some GSD-Ib patients. Divergent effects of HIF-1α on PPARG expression are described, with the ability of HIF-1α to either up- or downregulate PPARG in different cell types. Although the effects of PPARG activation have not, to our knowledge, been studied in neutrophils, in macrophages PPARG activation has been shown to be anti-inflammatory and to drive a phenotypic switch toward alternate activation.7,8 PPARG also regulates cellular metabolism, increasing glucose uptake and lipid anabolism. In cardiomyocytes, an HIF-1α and PPARG axis may act in concert to regulate cellular metabolism.9 It is interesting to speculate whether the amelioration of the functional defects in GSD-Ib patient neutrophils by inhibition of PPARG is mediated by effects upon cellular metabolism, and further studies to look at metabolite levels in patient cells following treatment with a PPARG antagonist would be of interest. In GSD-Ib patients, PPARG-stimulated fatty acid synthesis will be limited by availability of cytoplasmic acetyl coenzyme A as a consequence of defective glucose cycling, again raising the interesting question of whether it is the energetic context in which PPARG is induced that determines the consequences for neutrophil function.
A key message of this paper is that, in the context of impaired glucose uptake and intracellular cycling, defects in ATP generation cannot be rescued by enhanced HIF-1α expression. In parallel with the observations made for PPARG, this raises the possibility that the context in which HIF stabilization is observed may be critical in determining myeloid cell energetics and consequently their functional responses. This has broader relevance to neutrophil biology, outwith the setting of GSD-Ib disease, given that in the context both of host-pathogen interactions and chronic inflammation, the regions to which neutrophils are recruited are characterized by both nutrient and oxygen deprivation. Given the current paucity of effective therapeutic strategies for targeting neutrophilic inflammation and the profound consequences of ATP depletion for key neutrophil functions, dissecting the complex interactions between HIF oxygen-sensing pathways and metabolic flux is of fundamental importance in myeloid cell biology.
Conflict-of-interest disclosure: The authors declare no competing financial interests.