In this issue of Blood, Sapey et al1 report that the human polymorphonuclear neutrophil leukocyte (or neutrophil) undergoes an age-related loss of its ability to migrate up chemotactic gradients, a functional defect that seems causally related to alterations in the polyphosphoinositide pathway.2
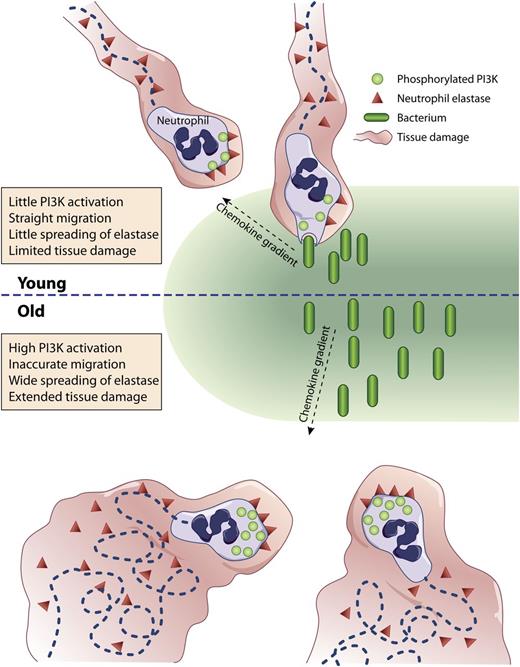
(Top) Neutrophils from young (<35 year old) individuals migrate directly up a chemotactic gradient toward an infectious focus where they phagocytose bacteria causing limited tissue injury from little release of elastase. (Bottom) Neutrophils from aged (>65 year old) individuals migrate haphazardly delaying their arrival to the infection site and causing extended tissue damage from the spreading of elastase.
(Top) Neutrophils from young (<35 year old) individuals migrate directly up a chemotactic gradient toward an infectious focus where they phagocytose bacteria causing limited tissue injury from little release of elastase. (Bottom) Neutrophils from aged (>65 year old) individuals migrate haphazardly delaying their arrival to the infection site and causing extended tissue damage from the spreading of elastase.
The decline in the efficiency of the immune system is a well-established consequence of aging, a phenomenon termed immunosenescence. The impact of aging on immunity has been principally addressed within the context of the acquired arm of the immune system,3 and little is known about the senescence of neutrophils. This is surprising considering that the neutrophil occupies a central role in the initiation and regulation of the inflammatory response and in the first line of defense of the organism against injurious stimuli. The neutrophil also regulates the effector arm of the immune system by virtue of its ability to synthesize and secrete a wide variety of cytokines and chemokines. In addition, its phenotypic plasticity allows it to express functional MHC class II molecules and possibly functional T-cell receptors. These functions of the neutrophil are not fully understood or appreciated.4 It is nevertheless clear that profound functional links exist between the neutrophil and the other actors of the immune response. Characterizing how aging impacts neutrophil functions is therefore critical if we hope to improve the immune response of the aged.
Neutrophils need to move to the site of tissue infection or injury to accomplish their functions. They are accordingly one of the most, if not the most, motile of blood cells. The ability of neutrophils to move up a chemotactic gradient in an amoeboid fashion, termed chemotaxis, allows them to be effective. In the early 1980s, Corberand et al reported the reduced chemotactic ability of neutrophils from older individuals.5 Now, >30 years later, the molecular mechanisms involved in this dysfunction begin to unravel.
In a methodical series of experiments, Sapey et al provide evidence that blood neutrophils isolated from a large sample of donors of different ages (21-89 years) gradually lose, not their intrinsic motility, but their ability to move accurately up a concentration gradient of the major defined chemotactic factors, as well as toward sputum collected from patients with confirmed Streptococcus pneumonia infection. A constitutive activation of phosphatidylinositol 3-kinase (PI3K) in neutrophils isolated from older (>65 year old) donors (as evidenced by the tyrosine phosphorylation of the p85 regulatory subunit of class IA PI3K) is described. The authors then show that, although inhibition of PI3K decreases the migratory ability of neutrophils from younger donors (<35 years old), it restores the young phenotypes to the neutrophils isolated from older donors. Conversely, inhibition of the 5-polyphosphoinositide phosphatase phosphatidylinositol-3,4,5-trisphosphate 5-phosphatase 1 responsible for turning off the PI3K pathway transforms young neutrophils into old ones and amplifies the old phenotype of old neutrophils. Subsequent inhibitor studies point to a specific role of a subset of PI3Ks, namely PI3Kδ and PI3Kγ, in these observations. The authors finally suggest that the inappropriate meandering of neutrophils from older donors is associated with increased membrane-associated (or shed) proteinase activity and degradation of tissues and/or extracellular matrices (see figure).
The description of the loss of the ability of neutrophils to properly orient themselves and migrate to the appropriate site is a highly significant observation in that this functional alteration is likely to impact profoundly on their ability to exercise their protective roles in the inflammatory and immune responses. An inappropriate neutrophil recruitment and activation will limit their ability to respond to infectious stimuli. It will also negatively affect their adeptness to properly orchestrate the subsequent immune response.
Based on their inability to restore the function of old cells with young plasma, the authors propose that the old neutrophils are intrinsically deficient. The short (45 minutes) incubation period in the young environment, however, might not be long enough to reverse the putative effect of chronic exposure to an old environment. Indeed, is the described higher activation level of PI3K (that led to disrupted chemotaxis) and the increased release of proteinases (resulting in greater tissue damage) the cause or the consequence of the augmented inflammatory status in the elderly? This is intriguing, and further work directly addressing this important question is needed to determine the intrinsic and extrinsic contributions to the functional defects of neutrophils.
The data described raise a number of fascinating questions for future investigations. What are the signaling elements upstream of the tyrosine phosphorylation of p85 that are affected by aging? What are the biochemical and downstream signaling consequences of the increases in phosphatidylinositol(1,4,5)-trisphosphate [PtdIns(1,4,5)P3; the product of the activity of PI3Ks] that are suggested by the data presented (this is a major limitation of the present studies as it has yet to be established)? What are the temporal and spatial distributions of PtdIns(1,4,5)P3 in resting and stimulated neutrophils [PtdIns(1,4,5)P3 transiently accumulates, among others, at the front of moving cells and at the base of the phagocytic cups during particle internalization (phagocytosis)]? What are the respective contributions of PI3Kδ and PI3Kγ? Why and how do the neutrophils lose their “compass” on hyperphosphorylation of p85? Is there a causative link between the dysregulation of the PI3K pathway and the increased proteinase activity? Does the altered regulation of the PI3K pathway lead to other phenotypic changes in neutrophils such as altered life span or the potential of acquiring the ability to interact with the other components of the immune system? Do similar events occur in other immune cells on aging and with what consequences? An affirmative answer to the latter question would indicate that treatments targeting the PtdIns(1,4,5)P3 pathway would have consequences on the immune system broader than just the restoration of migration accuracy of neutrophils.
By identifying specific PI3Ks involved in the ability of neutrophils to migrate appropriately, these studies go beyond a descriptive analysis of a phenomenon of basic, as well as clinical and societal, relevance. The reversal of the old phenotype by PI3K inhibitors suggests avenues of research for improving disease outcomes in aging patients.
Conflict-of-interest disclosure: The authors declare no competing financial interests.