In this issue of Blood, Horwitz et al present encouraging results of a phase 2 study assessing the efficacy of the antibody-drug conjugate (ADC) brentuximab vedotin (BV) in relapsed/refractory peripheral T-cell lymphoma (PTCL).1
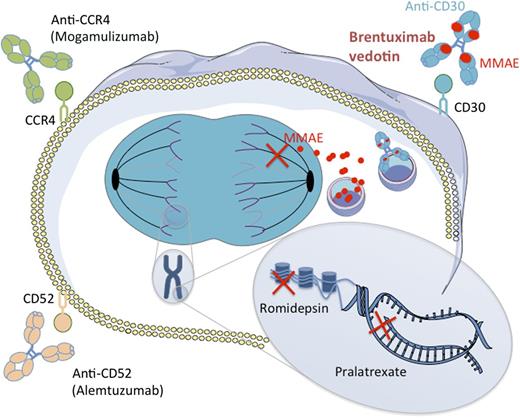
Mechanisms of action of new drugs in PTCL. Two naked antibodies targeting the CD52 and CCR4 molecules (alemtuzumab and mogamulizumab, respectively) have shown interesting potency in PTCL by mediating antibody-dependent cell-mediated and complement-dependent cytotoxicity. Romidepsin is an HDAC, which is able, among other mechanisms, to restore expression of proapoptotic genes. Pralatrexate is a dihydrofolate reductase inhibitor that impedes DNA synthesis. BV is an ADC combining an anti-CD30 monoclonal antibody attached by a protease-cleavable linker to a cytotoxic agent, the MMAE. MMAE is released upon internalization by CD30-expressing cells and disrupts microtubule polymerization. HDAC, histone deacetylase inhibitor.
Mechanisms of action of new drugs in PTCL. Two naked antibodies targeting the CD52 and CCR4 molecules (alemtuzumab and mogamulizumab, respectively) have shown interesting potency in PTCL by mediating antibody-dependent cell-mediated and complement-dependent cytotoxicity. Romidepsin is an HDAC, which is able, among other mechanisms, to restore expression of proapoptotic genes. Pralatrexate is a dihydrofolate reductase inhibitor that impedes DNA synthesis. BV is an ADC combining an anti-CD30 monoclonal antibody attached by a protease-cleavable linker to a cytotoxic agent, the MMAE. MMAE is released upon internalization by CD30-expressing cells and disrupts microtubule polymerization. HDAC, histone deacetylase inhibitor.
Except for anaplastic lymphoma kinase (ALK)-positive anaplastic large-cell lymphomas (ALCLs), PTCLs encompass a broad range of subentities that share a dismal prognosis. Compared with the major breakthrough associated with the use of anti-CD20 antibodies in the treatment of B-cell lymphomas, no major advances were made during the last decade in PTCL other than ALK+ ALCL. Hence, no immunotherapy such as rituximab was successfully developed in PTCL. Yet, interesting results came from the use of the anti-CD52 antibody alemtuzumab or the anti-CC chemokine receptor 4 (CCR4) antibody mogamulizumab. The efficacy of alemtuzumab was firstly suggested as a single agent in a pivotal phase 2 study of heavily pretreated patients with PTCL and was secondly confirmed in de novo PTCL in association with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) with an impressive complete response rate (71%).2 However, premature closures due to a high rate of opportunistic infections in other studies tempered the interest of alemtuzumab in combination with conventional chemotherapy.3 Mogamulizumab was associated with an interesting 50% overall response rate (ORR) in adult T-cell leukemia/lymphoma in which CCR4 is expressed at the cell surface. ORR was 35% in patients with CCR4+ relapsed PTCL or cutaneous T-cell lymphoma (CTCL) in a recently published phase 2 trial with reversible and manageable toxicity.4
Aside from immunotherapy, the US Food and Drug Administration (FDA) approved 2 drugs for patients with PTCL in relapse/refractory settings. In 2009, pralatrexate was approved, showing an ORR of 29% in 109 evaluable patients with refractory/relapsed PTCL in a phase 2 study5 ; in 2011, romidepsin was approved, showing an ORR of 25% among 130 patients in a similarly designed trial.6 Of note, recent data on bendamustine in refractory/relapsed PTCL and CTCL at 120 mg/m2 on days 1 and 2 every 3 weeks underlie an appealing efficacy with a 50% ORR.7 In all of these studies, progression-free survival (PFS) was short (<6 months) even if very long duration of response was observed in complete remission (CR) patients.
BV is an ADC combining the chimeric antibody SGN-30–targeting CD30 antigen, a member of the tumor necrosis factor receptor superfamily, and monomethyl auristatin E (MMAE), an anti-microtubule drug with potent cell-killing activity. Compared with SGN-30, impressive response rates were observed with its ADC counterpart BV in a phase 1 study for patients with Hodgkin lymphoma and ALCL in which the maximum tolerated dose (MTD) was defined as 1.8 mg/kg every 3 weeks (50% ORR for patients receiving the MTD).8 This high efficacy was confirmed in 2 pivotal, open-label, single-arm phase 2 trials for patients with refractory/relapsed Hodgkin lymphoma after autologous transplantation and for patients with refractory/relapsed ALCL, respectively (see figure).9
Given those findings, Horwitz et al conducted a phase 2 study evaluating the ORR for patients with relapse/refractory PTCL (not otherwise specified or angioimmunoblastic).1 Of note, this study is a planned subset analysis of BV efficacy assessment in relapse/refractory T- and B-cell lymphomas. Although the subset of patients with PTCL is quite small (n = 34) compared with previous phase 2 studies with pralatrexate (n = 115) or romidepsin (n = 131), and BV in Hodgkin lymphoma (n = 102) or ALCL (n = 58), the overall and CR rates (41% and 23%, respectively) in this relapse/refractory setting are of utmost interest.1 As with other drugs, PFS and duration of response were short (around 6 months). In addition, no unexpected or unmanageable toxicity was observed with neutropenia, and peripheral neuropathy was the most frequent adverse event as previously described with BV.
In line with previous reports with BV or other ADC, no correlation was found between CD30 expression at the tumor cell surface and clinical response. This emphasizes that selection of patients based only on quantitative antigen assessment on tumor cells is not mandatory. Several explanations have been proposed to elucidate this apparently paradoxical lack of correlation between ADC potency and target expression. A homogeneous expression of the targeted antigen does not seem a prerequisite for efficacy.10 Further mechanistic studies are warranted to fully understand such a lack of correlation.
The high potency and low toxicity of BV reported in the present study shed light on the possibility of combining the drug with conventional chemotherapy both in de novo and in relapse/refractory disease. However, a formal warning by the FDA was emitted concerning the use of BV and bleomycin due to a high rate of pulmonary toxicity. A phase 3 trial comparing BV-AVD (doxorubicin, bleomycin, vinblastine, and dacarbazine [ABVD] without bleomycin) and ABVD is currently proceeding (NTC01712490). Similarly, a phase 3 randomized trial (NCT01777152) comparing CHOP vs BV-CHP (CHOP without oncovin) for patients with de novo CD30+ PTCL is ongoing. In the near future, such combinations of BV with CHP in first line or with bendamustine in second or subsequent lines of treatment (and as a bridge toward stem cell transplant in some cases) might therefore refine the poor prognosis of these PTCL patients with a high unmet medical need.
Conflict-of-interest disclosure: B.C. has been on advisory boards for Celgene, Millennium, and Spectrum. The remaining author declares no competing financial interests.