In this issue of Blood, Arman et al show that bacteria use immunoglobulin G (IgG) from plasma to engage platelet surface receptors FcγRIIA and integrin αIIbβ3 to induce platelet activation, which is further facilitated by platelet factor 4 (PF4).1
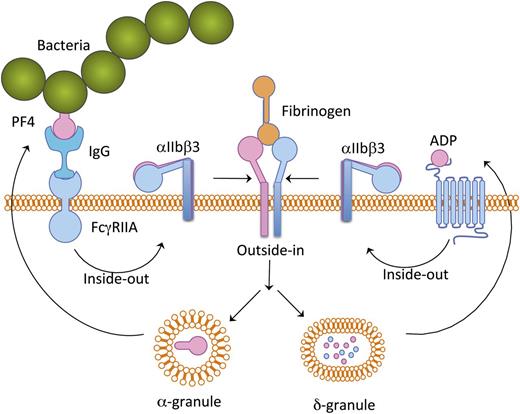
Schematic representation of platelet activation cascade induced by bacteria. Bacteria-bound PF4 is recognized by plasma IgG and this complex binds to platelet FcγRIIA receptor, thereby inducing a mild inside-out signaling leading to integrin αIIbβ3 activation. Fibrinogen binding to the activated αIIbβ3 results in granular secretion. ADP secreted from δ-granules provides positive feedback. PF4 secreted from α-granules potentiates further binding of additional bacteria.
Schematic representation of platelet activation cascade induced by bacteria. Bacteria-bound PF4 is recognized by plasma IgG and this complex binds to platelet FcγRIIA receptor, thereby inducing a mild inside-out signaling leading to integrin αIIbβ3 activation. Fibrinogen binding to the activated αIIbβ3 results in granular secretion. ADP secreted from δ-granules provides positive feedback. PF4 secreted from α-granules potentiates further binding of additional bacteria.
Blood platelets play an important role during physiological hemostasis and pathological thrombosis.2 On unactivated platelets, integrin αIIbβ3, the platelet fibrinogen (Fg) receptor, is in a low-affinity state, unable to bind soluble Fg. During platelet activation by agonists, a cascade of signaling events induces platelet shape change, granular secretion, and thromboxane A2 (TxA2) generation, ultimately resulting in a conformational change in the extracellular domains of αIIbβ3, thereby converting it into a high-affinity state capable of binding Fg, in a process known as inside-out signaling.3 Once Fg binds to the activated integrin αIIbβ3, a signal is once again transmitted, this time from the extracellular domain of αIIbβ3 through the transmembrane domain to the cytoplasmic domain, a phenomenon known as outside-in signaling.3 During inside-out signaling, platelet granules are secreted and TxA2 is generated which provides feedback to platelet activation and facilitates platelet recruitment. Activated platelets also provide a surface to assemble coagulation pathways that generates thrombin, which forms and stabilizes the fibrin clot.4
Historically, the function of platelets has been viewed as to initiate hemostasis. However, recent developments in platelet biology indicate that platelets harbor a number of proteins that are important for several functions that are primarily performed by other cells. These findings led to the appreciation of the role of platelets in tissue remodeling, inflammation, angiogenesis, immunity, and antimicrobial host defense.5-9 Bacteria have been shown to interact with platelets through their surface proteins, which are strain-specific.10 It is largely believed that this interaction of platelets with bacteria is the initial step of host defense. However, certain bacterial strains are capable of subverting this defense mechanism and exploiting it to their advantage. The mechanism through which these strains manipulate platelet function is largely unknown.
Arman and colleagues evaluated the effect of 5 bacterial strains belonging to the Staphylococcus and Streptococcus family on their ability to activate human platelets.1 They show that the interaction of bacteria with platelets requires the platelet FcγRIIA receptor because the inhibition of FcγRIIA by monoclonal antibody IV.3 blocks platelet activation. They also show the requirement of plasma IgG and engagement of integrin by fibrinogen for the bacteria to induce platelet activation. Furthermore, feedback agonists adenosine 5′-diphosphate (ADP) and TxA2 that are released from activated platelets are mandatory for platelet aggregation induced by bacteria. PF4 a constituent of α-granule binds to bacteria and reduces the lag time of bacteria-induced platelet aggregation. The essential role for PF4 in this process is further supported by the fact that platelets lacking PF4 as in gray platelet syndrome fail to aggregate in response to bacteria.
The finding of Arman et al has shed light on the mechanism of platelet activation by certain bacteria.1 Although bacteria in circulation have been shown to interact with platelets, probably through strain-specific proteins, certain bacterial strains may first bind to a small amount of PF4 present in circulation. The bacteria-associated PF4 is then recognized by circulating immunoglobulins (IgGs). This immunocomplex consisting of PF4 and bacteria binds platelet through a FcγRIIA receptor present on the platelet surface. This interaction of bacteria with platelets induces a mild signal within the platelets that results in an inside-out signaling leading to integrin αIIbβ3 activation. Fibrinogen present in the plasma then binds to the activated integrin and induces an outside-in signaling leading to secretion of α- and δ-granular contents as well as generation of TxA2. These secreted agonists provide a boost to the already-initiated platelet inside-out signaling, causing robust platelet aggregation. The PF4 secreted from the α-granules in turn binds more bacteria and the vicious cycle continues (see figure).
Recent discoveries indicate that platelets express Toll-like and purinergic receptors which help in detection of bacterial infection and release of a range of bactericidal molecules, thus contributing to the process of host defense toward infection.9 It has also been recognized that certain strains of bacteria interact with platelets and induce platelet aggregation. The work presented by Arman et al clearly provides a molecular mechanism in this regard.1 It appears that bacteria, through the virtue of the cellular machinery, exploit the platelet host defense mechanism to achieve improved virulence. Further work is warranted to show whether this bacteria-induced platelet activation is in fact advantageous to the pathogen.
Conflict-of-interest disclosure: The author declares no competing financial interests.