Key Points
Objective responses observed with brentuximab vedotin in 41% of patients with relapsed T-cell lymphomas, including 54% of AITL patients.
Objective responses observed across a wide range of CD30 expression, including undetectable CD30 expression per central review.
Abstract
This phase 2, open-label, multicenter study evaluated the efficacy and safety of brentuximab vedotin, a CD30-directed antibody-drug conjugate, in relapsed/refractory CD30+ non-Hodgkin lymphomas. The primary end point was objective response rate (ORR). Key secondary end points included safety, correlation of CD30 expression with response, response duration, and progression-free survival (PFS). Brentuximab vedotin 1.8 mg/kg was administered every 3 weeks until progression or unacceptable toxicity. This planned subset analysis included patients with peripheral T-cell lymphomas (PTCLs; n = 35), specifically angioimmunoblastic T-cell lymphoma (AITL; n = 13) and PTCL not otherwise specified (n = 22). Median age was 64 years; 63% were refractory to most recent therapy. Of 34 evaluable patients, ORR was 41% (8 complete remissions [CRs], 6 partial remissions [PRs]), and ORR was 54% in AITL (5 CRs, 2 PRs) with median PFS of 6.7 months thus far. No correlation between CD30 expression per central review and response was observed. Safety data were consistent with the known profile of brentuximab vedotin, and included at least grade 3 events of neutropenia (14%), peripheral sensory neuropathy, and hyperkalemia (9% each). In summary, brentuximab vedotin showed antitumor activity in patients with relapsed PTCL particularly AITL. This trial was registered at www.clinicaltrials.gov as #NCT01421667.
Introduction
Inadequate response, either relapse or inability to achieve a remission, remains a major problem in the management of patients with mature or peripheral T-cell lymphomas (PTCLs). In several studies of newly diagnosed patients with PTCLs, multiagent chemotherapy resulted in overall response rates (ORRs) ranging from 39% to 84%, with a low proportion of complete remissions (CRs).1-3 Long-term progression-free survival (PFS; 3-year and 5-year) was only 36% to 44% even in studies where high-dose therapy and autologous stem cell transplantation as consolidation of remission had been used.4,5
There remains a significant clinical need for new, active agents in both the frontline and relapsed settings.6 The historical outcomes for patients with relapsed disease have been especially dismal. In a recently published series describing the population-based experience of the British Columbia Cancer Agency (BCCA), Mak et al reported a median overall survival (OS) of only 5.5 months for patients with relapsed or refractory PTCLs who did not undergo transplant, highlighting the lack of available and effective therapies for these patients.7 In addition, the BCCA study showed that there was no statistically significant difference in OS after relapse between each of the PTCL subtypes: angioimmunoblastic T-cell lymphoma (AITL; 7.7 months), PTCL not otherwise specified (PTCL-NOS; 6.5 months), and anaplastic large cell lymphoma (ALCL; 3.0 months). As the BCCA series included patients diagnosed between 1976 and 2010, it does not capture possible gains from novel agents recently approved for T-cell lymphomas. In 2009, pralatrexate was approved with a 29% ORR in a phase 2 study of 115 subjects with a wide range of T-cell lymphomas.8 In a similarly designed phase 2 study of 131 patients, a 25% ORR with single-agent romidepsin resulted in an approval in 2011.9 For a specific subtype of PTCL, namely systemic ALCL, single-agent brentuximab vedotin treatment resulted in an 86% ORR and a 57% CR rate in relapsed or refractory disease, resulting in regulatory approval for this disease in 2011.10 Brentuximab vedotin is an antibody-drug conjugate (ADC) comprising an anti-CD30 antibody conjugated to monomethyl auristatin E (MMAE) that binds to human CD30. After binding to the cell surface, nonclinical data suggest that the ADC internalizes, then releases MMAE via proteolytic cleavage, and subsequently induces cell-cycle arrest and apoptotic death of the tumor cell.11 Of note, ALCL is characterized by uniform high CD30 expression on malignant cells, whereas other subtypes of PTCL have variable CD30 expression.12
The purpose of this study was to explore the activity of single-agent brentuximab vedotin in patients with non-Hodgkin lymphomas (NHLs) whose tumor expressed CD30 at any level. The primary objective of the study was to determine the antitumor activity of treatment with brentuximab vedotin as measured by the ORR. Key secondary objectives included safety, characterization of the relationship of CD30 expression with antitumor activity, duration of response, and PFS. The study enrolled both B- and T-cell lymphomas (excluding ALCL). This planned subset analysis presents data for patients with PTCLs enrolled on this study.
Methods
This phase 2, open-label, multicenter study was initiated to evaluate efficacy and safety of single-agent brentuximab vedotin in relapsed or refractory NHL, including both World Health Organization (WHO) classifications of B-cell and mature T-/NK-cell neoplasms (NCT01421667).13 The primary end point was ORR as determined by the investigator per the Revised Response Criteria for Malignant Lymphoma14 and key secondary end points included safety, correlation of CD30 expression with response, duration of objective response, and PFS. The planned subset analysis for patients enrolled with PTCLs is presented here.
Patient eligibility
Patients with T-cell lymphomas were enrolled between September 2011 and November 2012. Key eligibility criteria included histologically confirmed mature T-cell lymphoma with any detectable CD30 expression per institutional laboratory using immunohistochemical (IHC) staining with the BerH2 antibody clone on a biopsy of the most recent relapsed or refractory disease. Patients with Sezary syndrome, mycosis fungoides (including transformed mycosis fungoides), and ALCL were excluded. Eligible patients also had at least 1 prior systemic therapy, measurable disease, age ≥12 years, and Eastern Cooperative Oncology Group (ECOG) performance status of ≤2. Patients were required to have adequate baseline laboratory values, including an absolute neutrophil count ≥1000/µL, platelet count ≥50 000/µL, serum creatinine ≤1.5 times the upper limit of normal (ULN), aspartate aminotransferase and alanine aminotransferase ≤2.5× ULN, and serum bilirubin ≤1.5× ULN or ≤3× ULN for patients with Gilbert disease or documented hepatic involvement with NHL. In addition, previous allogeneic stem cell transplant was allowed only if patients were >100 days from stem cell transplant and did not have active acute or chronic graft-versus-host disease. Exclusion criteria included prior treatment with brentuximab vedotin and evidence of cerebral/meningeal disease or a history of progressive multifocal leukoencephalopathy. A minimum of 4 weeks since last systemic therapy was required, unless underlying disease was progressing on therapy.
Study design and treatment
Patients were treated with 1.8 mg/kg brentuximab vedotin IV on day 1 of each 3-week cycle. Patients who achieved at least stable disease (SD) were eligible to receive continued brentuximab vedotin treatment until disease progression, unacceptable toxicity, or study closure. Patients who received at least 1 dose of brentuximab vedotin were followed for disease status and survival.
Routine premedication was not allowed for the prevention of infusion-related reactions prior to the first dose of brentuximab vedotin; however, patients who experienced a grade 1 or 2 infusion-related reaction could receive subsequent study treatment infusions with premedication consisting of acetaminophen and diphenhydramine. Patients with grade 3 or 4 infusion-related reactions were allowed to receive additional brentuximab vedotin treatment at the discretion of the investigator. Intrapatient dose reduction to 1.2 mg/kg brentuximab vedotin was allowed depending on the type and severity of toxicity. Dose delays of up to 3 weeks were allowed for resolution of toxicity. Use of platelet and/or red blood cell transfusion or granulocyte colony-stimulating factors was allowed during study. Low-dose prednisone (≤20 mg per day) was allowed; however, steroid use in higher doses or as an antineoplastic agent was prohibited.
The study was approved by each site’s institutional review board, and written informed consent was obtained from all enrolled patients prior to study-specific procedures, per the Declaration of Helsinki. Data analyzed include data collected through September 2013.
Study assessments
Disease response was assessed by the investigator according to the Revised Response Criteria for Malignant Lymphoma.14 Clinical response of progressive disease (PD), SD, partial remission (PR), or CR was determined at each assessment. PD included PD per Cheson et al14 and clinical disease progression per the investigator. Computed tomography (CT) and positron emission tomography (PET) scans were required for all patients at baseline. If disease was not PET-avid at baseline, restage assessments were performed using CT scans of diagnostic quality. For patients with PET-avid disease at baseline, both PET and CT scans were required until disease was PET-negative; then, CT scans of diagnostic quality were used for subsequent restaging. Restaging assessments were performed at cycles 2, 4, every 3 cycles thereafter (between days 15 and 21), and at end of treatment. Cutaneous lesions were monitored via physical examination. If the bone marrow was positive at baseline, a follow-up bone marrow aspirate and biopsy was required and had to be negative for assessment of CR.
All patients who came off treatment were subsequently followed for disease status and survival every 3 months for the first 2 years and according to the institutional standard of care thereafter until death, study closure, or withdrawal of consent. Patients who discontinued study drug for any reason other than disease progression or initiation of a nonprotocol therapy for treatment of lymphoma had restaging scans every 6 months during the first year after the last dose of brentuximab vedotin and with respect to the institutional standard of care thereafter.
Safety assessments included surveillance and recording of adverse events (AEs), physical examination findings, and laboratory tests. AE severity was graded using the National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI CTCAE), version 4.0.3. Blood concentrations of brentuximab vedotin ADC and MMAE, as well as total antibody and antitherapeutic antibodies to brentuximab vedotin, were measured. Assessment of CD30 expression to determine eligibility was performed by institutional laboratories; tissue samples were also sent to the central pathology laboratory (Quest Diagnostics) for subsequent evaluation of CD30 expression using standard IHC and the BerH2 antibody. For consistency, the central CD30 result is reported here. The central laboratory created new slides from each tumor specimen, had a standardized method of staining, and had a consistent definition of CD30 expression, using only CD30 expression on neoplastic cells. Soluble CD30 (sCD30) concentrations were measured in a commercial bead-based sandwich fluoroimmunoassay modified to eliminate interference by brentuximab vedotin; assays were performed on a Bio-Plex 200 system (Bio-Rad Laboratories, Inc.) with appropriate calibrators and controls.
Statistical analysis
Patient disposition, demographics, disease characteristics, safety, disease response, and exposure to study drug were analyzed by disease diagnosis (AITL and PTCL-NOS), as well as for total T-cell neoplasms. The primary end point of ORR was defined as the proportion of patients with CR or PR as best clinical response according to the Revised Response Criteria for Malignant Lymphoma. The primary efficacy hypothesis test (ORR ≤10% vs ORR >10%) was planned for the WHO NHL classification of mature T-/NK-cell neoplasms. Based on the study design, the null hypothesis would be rejected if 7 (23% ORR) or more of the 30 planned patients with that NHL classification respond with 1-sided α of 0.05. With a 29% ORR, the study would provide an 81% power for the relapsed or refractory CD30-positive mature T-/NK-cell neoplasm cohort.
Key secondary end points included safety, correlation of CD30 expression with response, duration of objective response, and PFS. The correlation of CD30 expression with response was assessed using Loess.15 Duration of response is defined as the time from start of the first documentation of objective tumor response (CR or PR) to the first documentation of tumor progression or to death due to any cause. PFS was defined as the time from start of study treatment to first documentation of tumor progression or to death due to any cause and was estimated using Kaplan-Meier survival methodology. Tumor progression included both radiologic evidence of progression and/or a determination of clinical progression per investigator. The pharmacokinetic (PK) parameters of brentuximab vedotin, MMAE, and total antibody were estimated and summarized with descriptive statistics.
Results
Patients
Thirty-five patients with mature T-cell lymphomas with variable CD30 expression as determined by a local institution were enrolled and treated at 13 sites in the United States and 1 site in Canada. Patient demographics, baseline disease characteristics, and prior cancer-related therapies are summarized in Table 1. Diagnoses included AITL (n = 13) and PTCL-NOS (n = 22). Median age was 64 years (range, 33-83 years) and most patients were male (77%) and white (83%). The majority of patients entering the study had an impaired ECOG performance status of 1 or 2 (80%) and most had stage III or IV disease (77%). The median number of prior therapies was 2 (range, 1-9) and 63% were refractory to their most recent therapy. Eleven patients (31%) had never achieved an objective response with any prior therapy.
Based on the eligibility criteria, all patients had lymphoma that expressed CD30 by institutional pathology assessment; however, 6 (17%) had undetectable CD30 expression by central pathology assessment. CD30 expression per central laboratory assessment ranged from 0% to 95%. Two patients (6%) had missing results and 1 patient (3%) could not be assessed for CD30 positivity by central review because the tissue was inadequate for evaluation. All patients had elevated sCD30 at baseline ranging from 89 ng/mL to greater than the upper limit of quantitation (>14 320 ng/mL) compared with the normal range (≤29 ng/mL). Median baseline sCD30 for patients with AITL and PTCL-NOS was 1214 ng/mL (range, 108-9473 ng/mL) and 840 ng/mL (range, 89 to >14 320 ng/mL), respectively.
One patient with PTCL-NOS was excluded from the efficacy-evaluable analysis set because no postbaseline restaging assessment was performed nor a determination of clinical progression per the investigator. The patient discontinued treatment after 1 dose due to an AE of grade 1 pyrexia.
Efficacy
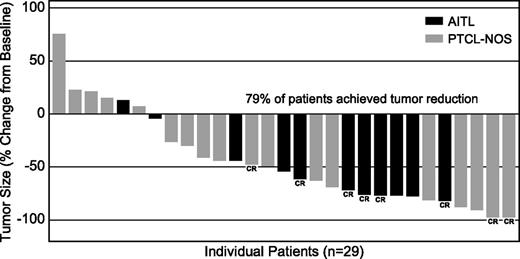
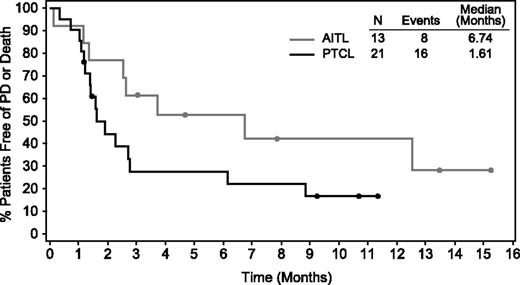
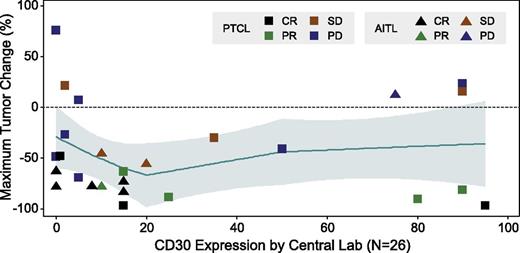
Of the 34 evaluable patients with mature T-cell lymphomas, 14 (41%) achieved an objective response (8 CR, 6 PR) (Table 2). Over half of the patients with AITL (54%) achieved an objective response, including 5 CRs and 2 PRs, and one-third (33%) of the PTCL-NOS patients achieved an objective response (3 CRs, 4 PRs). Five of the 34 evaluable patients did not have postbaseline imaging results; all were determined to have clinical disease progression per the investigator. Of the 29 patients with repeat imaging, 79% (n = 23) had postbaseline disease regression (Figure 1). To date, median duration of response for all patients was 7.6 months (range, 1.3-14+ months), 5.5 months (range, same as all) for AITL patients, and 7.6 months (range, 1.4-10.1+ months) for PTCL-NOS patients. Five responding patients remain in follow-up and 5 remain on therapy. Median duration of CR for all T-cell patients and for each subtype had not been reached at the time of this analysis (range for all patients and AITL, 1.8+ to 14+ months; range for PTCL-NOS, 7.6-10.1+ months). The median follow-up time from first dose was 2.7 months (range, 0.3-17.3 months). Thus far, the median PFS for all T-cell lymphoma patients was 2.6 months; however, 10 evaluable patients were progression free at >6 months from first dose of the study treatment. Those with AITL showed a median PFS of 6.7 months to date (range, 0.1-15.2+ months) and those with PTCL-NOS showed a median PFS of 1.6 months (range, 0.3-11.3+ months) (Figure 2). There was no apparent correlation between response and CD30 expression (Figure 3), as evidenced by complete or PRs in 9 of 14 patients (64%) with little to no detectable CD30 expression (≤15% CD30 expression) by central review.
Maximum tumor size reduction from baseline. Includes patients with postbaseline CT assessments (n = 29). Five of the 34 evaluable patients did not have postbaseline imaging; all were determined to have clinical disease progression.
Maximum tumor size reduction from baseline. Includes patients with postbaseline CT assessments (n = 29). Five of the 34 evaluable patients did not have postbaseline imaging; all were determined to have clinical disease progression.
PFS by histology subtype. PFS was analyzed using Kaplan-Meier methodology. Patients who were censored are indicated by a dot on the line.
PFS by histology subtype. PFS was analyzed using Kaplan-Meier methodology. Patients who were censored are indicated by a dot on the line.
Maximum tumor size decrease by quantitative CD30 expression. Includes patients who have both postbaseline radiographic response assessments and CD30 expression data. Loess methodology was used.
Maximum tumor size decrease by quantitative CD30 expression. Includes patients who have both postbaseline radiographic response assessments and CD30 expression data. Loess methodology was used.
Safety
A total of 35 patients received at least 1 dose of brentuximab vedotin and 5 patients (14%) remain on treatment. The median number of cycles received to date was 3 (range, 1-21) and the median time on treatment was 9 weeks (range, 2-78 weeks). As expected, duration of treatment was longer for responding patients with a median of 8.5 cycles of treatment (range, 4-21) and a median of 26 weeks (range, 12-78 weeks). Patients discontinued treatment due to PD (57%), AE (20%), and investigator decision (9%). AEs leading to treatment discontinuation included peripheral sensory neuropathy (6%), acute respiratory distress syndrome (ARDS), encephalopathy, Pneumocystis jiroveci pneumonia, pyrexia, and sepsis (3% each).
The safety data are consistent with those from historic data for brentuximab vedotin in relapsed systemic ALCL10 as represented by the most frequently occurring at least grade 3 events of grade 3 neutropenia (14%) and peripheral sensory neuropathy (9%). The only other at least grade 3 AE occurring in >2 patients was hyperkalemia (9%) (Table 3). Six treatment-emergent grade 4 AEs occurred in a total of 3 patients and included pneumonia, sepsis, hyperkalemia, lipase increased, confusional state, and pulmonary edema. Treatment-related serious AEs occurred in 4 patients and included pyrexia, rash, pneumonia (each grade 3), and ARDS (grade 5).
A total of 3 patients died within 30 days of the last dose. A 74-year-old patient with refractory AITL died due to ARDS related to disease progression, infection, and study treatment. The 2 other patient deaths were related to disease progression. One additional patient with SD had severe complications including sepsis (unrelated to treatment) that led to death ∼33 days after the last dose of brentuximab vedotin.
PK analyses
PK parameters for individual patients were determined using concentrations of serum brentuximab vedotin ADC and plasma MMAE, and actual sampling times relative to the start of infusion. The estimated area under the plasma concentration-time curve, maximum concentration, and time to reach maximum concentration of brentuximab vedotin and MMAE were consistent with those from historic data for brentuximab vedotin.10,16 Based on current analyses, there did not appear to be a significant correlation between response and exposure of ADC or MMAE.
Discussion
CD30 is expressed to varying degrees on many subtypes of T-cell lymphoma. In data obtained from a study characterizing cluster of differentiation (CD) markers in the disease of 319 patients with T-/NK-cell lymphomas, CD30 expression ranged from 0% to 64% in a variety of T-cell lymphomas including AITL and PTCL-NOS.12 In the subset of patients with T-cell lymphomas in this phase 2 study of the CD30-targeted ADC brentuximab vedotin, 41% of all evaluable patients responded, with 24% achieving a CR. At ∼6 months after the first dose of brentuximab vedotin, 10 of the 14 responders were progression free.
Responses were seen among patients with all levels of CD30 expression on their tumor samples, including 2 patients with undetectable CD30 by IHC on central review. This observation is consistent with preliminary results from a study presented by Krathen et al showing a lack of correlation of response to brentuximab vedotin with CD30 expression in patients with cutaneous T-cell lymphomas.17 In that study, 19 patients with mycosis fungoides were treated with brentuximab vedotin resulting in an ORR of 68%. Patients with low CD30 expression, defined in that study as <10% expression of lymphoid infiltrate, responded no less frequently than intermediate or high expressers. Quantitative image analysis (Nuance imaging system, CRi) showed that some of the samples from those that had undetectable CD30 expression on routine IHC were CD30-positive using this more sensitive technique. Interestingly, all patients on the study reported here had elevated sCD30 at baseline regardless of CD30 expression by IHC in tumor specimens, indicating the potential heterogeneity of CD30 expression and/or limitations of IHC to detect very low levels of CD30. Another hypothesis to explain the lack of correlation of response with CD30 expression may be local release of free drug from dying cells at an adequate concentration to kill adjacent tumor cells, even when a minority of the cells in a tumor express the CD30 target. The definitive explanation as to the lack of correlation between target and effect is not yet understood. The response rates in this trial and the lack of correlation with CD30 expression suggest potentially broad activity of brentuximab vedotin among various subtypes of T-cell lymphomas. Nevertheless, IHC for CD30 or presence of elevated sCD30 may be a useful biomarker in which to select patients; further study is warranted.
In terms of response, the ORR in this trial is comparable to that in similar populations studied with other recently approved agents, such as pralatrexate and romidepsin. The ORR of 41% in this trial places brentuximab vedotin among the active agents for PTCL. One key difference between this trial and the other trials was that a smaller number of patients were enrolled and analyzed (N = 115 and N = 131, respectively, vs N = 34); however, the median number of prior therapies, n = 2 (n = 3, pralatrexate; n = 2, romidepsin) and the frequency of patients who were refractory to their most recent prior therapy, 63% (63%, pralatrexate; 38%, romidepsin) were comparable to those in the larger phase 2 studies.8,9 The primary differences between this study population and those in the pivotal trials of pralatrexate and romidepsin were the omission of patients with ALCL and the requirement for some degree of CD30 expression. Newly diagnosed ALCL, in general, has a better prognosis than PTCL-NOS or AITL; however, whether ALCL maintains a better prognosis compared with PTCL-NOS or AITL after relapse remains controversial. A recent study by Mak et al indicated that this advantage is lost at the time of relapse,7 although another study by Smith et al suggested continued superior OS in relapsed ALCL patients compared with patients with other histologies.18 The lack of correlation with CD30 expression and response, and the lack of a known prognostic importance of CD30 expression in PTCL outside of high CD30 expression being an adverse factor in PTCL-NOS19 suggest that subjects on this trial do not represent a more favorable subset of PTCL. Furthermore, brentuximab vedotin was generally well tolerated in this study with no new safety signals detected in patients treated up to 21 cycles. Based on the activity and safety observed in patients on this investigational phase 2 trial, single-agent brentuximab vedotin has the potential to be a therapeutic option in treating relapsed and refractory PTCL.
Beyond the relapse setting, brentuximab vedotin has been safely combined at full dose with standard chemotherapy agents cyclophosphamide, hydroxydoxorubicin, and prednisone (CHP) for the frontline treatment of systemic ALCL and other CD30+ mature T/NK-cell lymphomas in a promising phase 1 trial.20 The results from the current phase 2 trial in PTCL coupled with the ability to combine brentuximab vedotin with standard induction chemotherapy has led to the initiation of a phase 3, double-blinded randomized study of brentuximab vedotin plus CHP vs CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) for untreated patients with CD30-expressing mature T-cell lymphomas.
Presented in part at the 13th International Conference on Malignant Lymphoma, Lugano, Switzerland, June 2013.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors wish to acknowledge Yin Yang for statistical guidance and Tiffany Griffin for assistance in manuscript preparation, both as employees of Seattle Genetics, Inc.
This work was sponsored by Seattle Genetics, Inc.
Authorship
Contribution: S.M.H. contributed to the analysis and interpretation of data and wrote the manuscript; R.H.A., N.L.B., E.D.J., J.P.S., O.A.O., T.S., and Y.O. contributed to the acquisition of the data and critically reviewed the manuscript; D.A.K. contributed to the analysis and interpretation of the data and critically reviewed the manuscript; and all authors contributed to the concept and design of the study and approved the final manuscript.
Conflict-of-interest disclosure: Seattle Genetics, Inc. provided research funding to the institutions of S.M.H., R.H.A., N.L.B., E.D.J., J.P.S., O.A.O., T.S., and Y.O.. T.S. and O.A.O. have acted as a consultant for Seattle Genetics, Inc. T.S. has participated in a Seattle Genetics, Inc. speakers’ bureau. Seattle Genetics, Inc. has provided N.L.B. and J.P.S. with funds for travel expenses. R.H.A., N.L.B., E.D.J., and T.S. have acted in an advisory capacity for Seattle Genetics, Inc. D.A.K. is an employee of and has equity ownership in Seattle Genetics, Inc.
Correspondence: Steven M. Horwitz, Department of Medicine, Lymphoma Service, 1275 York Ave, New York, NY 10021; e-mail: horwitzs@mskcc.org.