Key Points
The type of antibody secreted at relapse can serve as a marker of clonal heterogeneity.
It is important to monitor for serum FLC in the suspicion of clinical relapse to ensure that FLC relapse is not missed.
Abstract
Intraclonal heterogeneity was recently described in multiple myeloma (MM), but its full impact on disease progression and relapse has not been entirely explored. The immunoglobulin type produced by myeloma cells provides an excellent marker to follow changes in clonal substructure over time. We have prospectively evaluated serial paraprotein and serum free light chain (FLC) measurements and found that 258 of 520 and 54 of 520 patients who presented with a whole paraprotein relapsed with paraprotein only (PO) and “FLC escape,” respectively. The median overall survival of PO patients was longer, when compared with patients whose relapse manifested as an increase in FLC both alone and with a whole paraprotein, as a result of a significantly shorter survival from relapse of the latter groups. These observations fit a model in which 1 clone is able to produce a complete antibody, whereas the other secretes only FLC; the type of relapse represents the outgrowth of different clones, some of which are more resistant to therapy. To our knowledge, this is the largest series describing patients who have relapsed with FLC escape and highlights the importance of monitoring FLC when there is a suspicion of clinical relapse. This study was registered at www.isrctn.org as ISRCTN68454111.
Introduction
Although it has long been known that multiple myeloma (MM) is characterized by the proliferation of an abnormal clone of plasma cells, in more recent times the presence of intraclonal heterogeneity has been recognized.1-3 It is proposed that intraclonal heterogeneity, combined with the selective growth advantage conferred by additional genetic lesions, is an essential prerequisite for disease progression and relapse after chemotherapy. However, the full clinical impact of such heterogeneity has yet to be completely evaluated and it is of primary importance to understand its clinical implications. A tool which can be used to assess this question is the clonal secretion of immunoglobulin, an antibody known as paraprotein.1,4
Immunoglobulins are composed of 2 identical heavy chains and 2 identical light chains. The light chains are either κ, encoded on chromosome 2, or λ, encoded on chromosome 22. Heavy chains are encoded on chromosome 14 by a cluster of immunoglobulin heavy chain C-region genes for the production of the 5 classes and subclasses of immunoglobulin: IgM, IgD, IgG1-4, IgA1-2, and IgE.5 B cells and immunoglobulin-secreting plasma cells manufacture about twice as many light chains in their cytoplasm as heavy chains, which prevent toxicity to the cell from aggregation of free heavy chain.6,7 Normal as well as neoplastic plasma cells secrete both whole immunoglobulin and free light chains (FLCs). In a series of 2709 Medical Research Council (MRC) myeloma trial patients, 85% secreted a whole paraprotein (56% IgG, 27% IgA, and 2% IgD). In 70% of these whole paraprotein secretors, sufficient FLCs were secreted to exceed the renal threshold and become detectable in urine; in over 90%, FLC secretion was sufficient to perturb the normal serum FLC ratio. The remaining 13% of the total cohort secreted only FLC and 2% were oligo/nonsecretory.8
In recent years, in addition to the assessment of paraprotein levels in serum and urine, the diagnostic workup of MM has seen the introduction of serum FLC assays that can also be routinely used to both diagnose and monitor changes in disease activity.9-13 We have provisionally noted subclonal differences in heavy and light chain production within the predominant myeloma clones14 and, as such, the type of immunoglobulin produced can provide an excellent marker of clonal change over time. To better understand the extent of subclonal evolution over time as well as its impact on outcomes following relapse, we have prospectively evaluated serial FLC and paraprotein measurements in a large population of newly diagnosed MM patients treated within the Myeloma IX clinical trial.
Materials and methods
The MRC Myeloma IX trial (ISRCTN68454111) enrolled 1960 patients and the full design and primary results of the trial have been reported previously.15,16 In summary, the trial randomized newly diagnosed MM patients to receive thalidomide- vs nonthalidomide-containing therapy; thalidomide could be given both as an induction and/or as a maintenance regimen. Patients were divided between an intensive and a nonintensive pathway based on their eligibility for autologous stem cell transplantation. Primary end points included progression-free survival (PFS), overall survival (OS), and response. FLC and paraprotein levels were evaluated at diagnosis, after induction, and at relapse in all patients. The International Myeloma Working Group (IMWG) uniform response criteria12 were used to assess response and relapse in this study based on central laboratory analysis of serial blood and urine samples. Patients were classified as relapsing with FLC-only (FLC) escape if they failed to meet the IMWG criteria for change in paraprotein levels that define relapse but satisfied IMWG criteria for changes in FLC levels (Table 1). Statistical analysis was performed using SPSS version 20.0 (SPSS Inc.) and GraphPad Prism 6. Statistical significance of the differences between light chain and paraprotein levels was assessed using Mann-Whitney U tests. Survival curves were plotted using the Kaplan-Meier method. Differences between curves were tested for statistical significance using the log-rank test. Median follow-up was 5.9 years. The study was approved by the National Research Ethics Service London Surrey Borders Research Ethics Committee (reference 08/H0806/98), and was conducted in accordance with the Declaration of Helsinki.
Results
Centralized laboratory results were available for 647 patients with either IgG or IgA myeloma; of these, 32% (206 of 647) achieved a complete response (CR) as maximum response, of which 51% (106 of 206) were in stringent CR (sCR). Breaking this data set down further, in intensively treated patients, 187 of 428 (44%) achieved a CR with 94 of 187 (50%) being in sCR. In the nonintensively treated patients, 19 of 218 (9%) obtained a CR as maximum response, and 12 of 19 (63%) a sCR. To date, 520 of these 647 patients (80.5%) have relapsed. A significant increase in both paraprotein and FLC levels (paraprotein and light chain [PLC] relapse) was observed in 35.2% (183 of 520), while 258 of 520 (49.6%) relapsed with a significant increase only of their paraprotein levels (paraprotein only [PO] relapse) and 25 had relapse detected clinically. In 54 of 520 patients (10.4%), the relapse was characterized by an increase in FLC without a corresponding increase in paraprotein level, a phenomenon termed “serum FLC escape”17 ; these patients represented 6.5% (24 of 369) of IgG patients and 19.9% (30 of 151) of IgA patients, respectively. In 46 of 54 patients (85%), the increase in involved serum FLC was >200 mg/L, that is the level of increase recommended for defining relapse requiring treatment in the absence of clinical symptoms.18 In these 54 FLC escape patients, only 28 (51.8%) had a >200 mg/L increase in urine FLC levels. The definition of the different types of relapse and patients’ characteristics at diagnosis are detailed in Tables 1-2.
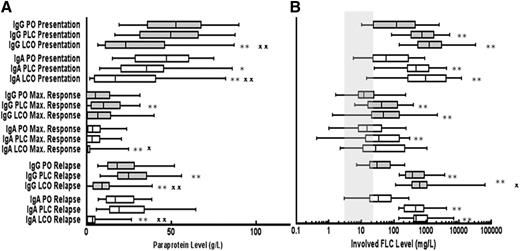
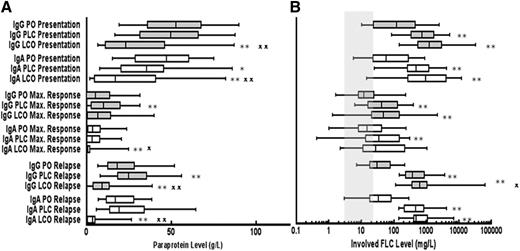
The paraprotein levels at presentation were significantly lower in both IgG and IgA patients who relapsed with FLC escape (LCO) as compared with patients who relapsed with a PLC relapse or PO (Figure 1A). The FLC levels at presentation and relapse were significantly higher in patients who relapsed with FLC escape compared with those relapsing with PO relapse (Figure 1B). Similarly, the relapse FLC levels were significantly higher for patients with IgG FLC escape relapse compared with patients with IgG PLC relapse but there was no significant difference between these groups for patients with IgA myeloma (Figure 1B).
Levels of serum paraprotein and FLC at presentation, maximum response, and relapse for patients grouped according to type of relapse. Whisker box plots showing the median, 25th, and 75th centiles for (A) paraprotein and (B) absolute levels of the involved light chain at presentation, maximum response, and relapse; tails represent 5th and 95th centiles (IgG PO, n = 186; IgG PLC, n = 138; IgG LCO, n = 24; IgA PO, n = 72; IgA PLC, n = 45; and IgA LCO, n = 30). Mann-Whitney U tests were performed to assess the statistical significance of the differences between the groups. *P < .05 when compared with PO at the same time point; **P < .01 when compared with PO at the same time point; X, P < .05 LCO vs PLC at the same time point; XX, P < .01 LCO vs PLC at the same time point. Shaded column represents normal range for sFLC. PO, paraprotein only; PLC, paraprotein plus FLC; LCO, FLC only.
Levels of serum paraprotein and FLC at presentation, maximum response, and relapse for patients grouped according to type of relapse. Whisker box plots showing the median, 25th, and 75th centiles for (A) paraprotein and (B) absolute levels of the involved light chain at presentation, maximum response, and relapse; tails represent 5th and 95th centiles (IgG PO, n = 186; IgG PLC, n = 138; IgG LCO, n = 24; IgA PO, n = 72; IgA PLC, n = 45; and IgA LCO, n = 30). Mann-Whitney U tests were performed to assess the statistical significance of the differences between the groups. *P < .05 when compared with PO at the same time point; **P < .01 when compared with PO at the same time point; X, P < .05 LCO vs PLC at the same time point; XX, P < .01 LCO vs PLC at the same time point. Shaded column represents normal range for sFLC. PO, paraprotein only; PLC, paraprotein plus FLC; LCO, FLC only.
A difference in survival between IgG and IgA myeloma patients has been previously established and reflects the short duration of remission in IgA patients.8 In this study, PFS and OS from relapse are 24 vs 20 months (P = .003) and 33.6 vs 28.6 months (P = .071) for IgG and IgA patients, respectively.
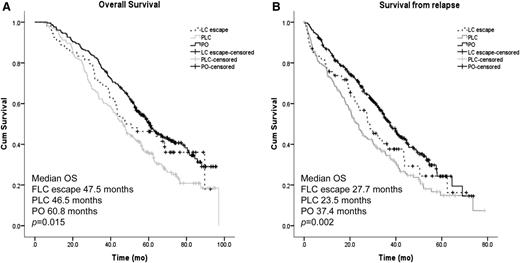
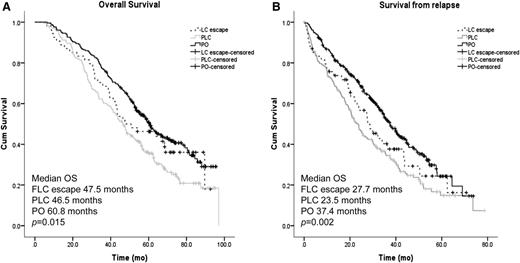
PFS was similar between all relapse groups (21.9 vs 18.0 vs 20 months for FLC escape, PLC, and PO relapse, respectively; P = .766). Conversely, the median OS of patients relapsing with light chain involvement (either FLC escape or PLC) was ∼13 months shorter compared with patients relapsing with a whole paraprotein (PO) (Figure 2A, P = .015); this was mostly attributable to a significantly shorter survival from relapse (27.7 vs 23.5 vs 37.4 months, P = .002, for FLC escape, PLC, and PO, respectively; Figure 2B).
Survival according to paraprotein and FLC secretion at first relapse. (A) Kaplan-Meier curves of OS from diagnosis for patients relapsing with whole PO, both PLCs, or patients with FLC escape phenomenon. (B) Kaplan-Meier curves of survival from first relapse for patients relapsing with whole PO, both PLCs, or patients with FLC escape phenomenon.
Survival according to paraprotein and FLC secretion at first relapse. (A) Kaplan-Meier curves of OS from diagnosis for patients relapsing with whole PO, both PLCs, or patients with FLC escape phenomenon. (B) Kaplan-Meier curves of survival from first relapse for patients relapsing with whole PO, both PLCs, or patients with FLC escape phenomenon.
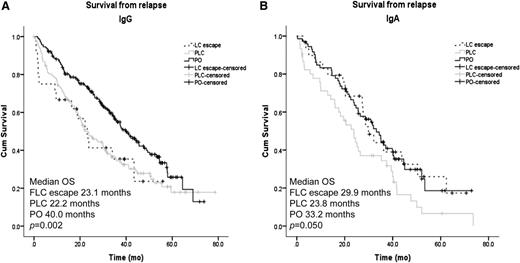
Examining the OS from diagnosis and first relapse by paraprotein isotype, IgG patients with PO relapse had a significantly improved OS from diagnosis (64.5 vs 43.4 and 47.3 months for FLC escape and PLC, respectively; P = .007). There was a trend toward increased OS from diagnosis for IgA patients who relapsed with PO, although this failed to reach statistical significance (50.9 vs 50.7 vs 40.2 months for PO, FLC escape, and PLC, respectively; P = .066). Survival from relapse was increased for patients relapsing without FLC involvement in both isotype subgroups (median OS from relapse 40 and 33.2 months for IgG and IgA, respectively) compared with patients in whom relapse was characterized by an increase in the involved FLC level (median survival from relapse 23.1 and 22.2 months for IgG FLC escape and PLC, and 29.9 and 23.8 months for IgA FLC escape and PLC, respectively; Figure 3A-B).
Survival from relapse according to paraprotein and FLC secretion at relapse for patients with IgG and IgA paraproteins. (A) Kaplan-Meier curves of survival from first relapse for IgG patients relapsing with whole PO, both PLCs, or patients with FLC escape phenomenon. (B) Kaplan-Meier curves of survival from first relapse for IgA patients relapsing with whole PO, both PLCs, or patients with FLC escape phenomenon.
Survival from relapse according to paraprotein and FLC secretion at relapse for patients with IgG and IgA paraproteins. (A) Kaplan-Meier curves of survival from first relapse for IgG patients relapsing with whole PO, both PLCs, or patients with FLC escape phenomenon. (B) Kaplan-Meier curves of survival from first relapse for IgA patients relapsing with whole PO, both PLCs, or patients with FLC escape phenomenon.
A Cox regression analysis including maximum response, age, paraprotein isotype, treatment pathway, thalidomide therapy, and the type of relapse identified a maximum response (at least very good partial response [VGPR]), an IgG paraprotein, intensive treatment, and the type of relapse as variables independently associated with an extended OS; treatment pathway and the type of relapse were also found to be variables associated with a longer survival from first relapse (Table 3).
Discussion
Many tumors, including MM, are traditionally thought to develop through a multistep process, via the acquisition of sequential “genetic hits.”19 The results of recent analyses have suggested that cancer is more likely to develop following a branching evolution pattern, typical of the model used by Darwin to explain the evolution of the species rather than in a linear stepwise fashion.2,20-22 Intraclonal heterogeneity, the essential substrate for Darwinian evolution, is present in myeloma but to date only limited information is available to judge the global impact of this phenomenon on disease progression and relapse. In a previous study aimed at understanding clonal evolution, a single-nucleotide polymorphism–based genome mapping tool was used to study 28 paired presentation-relapse samples. Three types of relapse were noted: (1) relapse with no additional genetic change; (2) relapse with additional molecular markers; and (3) relapse consistent with relapse from a precursor clone with differing molecular features to those detected at presentation.23
Since the finding that intraclonal heterogeneity is an important feature in MM, a number of studies have biologically evaluated this issue using different molecular and genetic approaches.2,3,23-26 However, the clinical impact that intraclonal heterogeneity may have on patients’ outcome is still not completely understood. Furthermore, it would be important for clinicians to have a quick and easy tool that allows them to assess the presence of intraclonal heterogeneity from a clinical perspective. Monitoring for the type of immunoglobulin produced and secreted at relapse (either a whole immunoglobulin or just a light chain) provides an excellent tool to study the global impact of intraclonal heterogeneity. The basic premise in this model is that patients harbor different clones with different secretory behavior,14 that is, 1 clone is able to produce a complete antibody, whereas the other secretes only an FLC and this can be used as a marker for subclonal progression. There is great heterogeneity in the patterns of monoclonal immunoglobulin types and levels secreted by the myeloma clones of different patients at presentation, before therapy has been administered. This was clearly shown by the observation that patients in the different groups, despite having similar proportions of κ (61%-67%) and λ subtypes, had very different levels of FLCs and paraprotein since presentation (Figure 1; Table 2). As expected, the groups with higher FLC levels at presentation (FLC escape and PCL) had a higher proportion of patients in whom light chains were detectable in the urine at presentation (Table 2). Within individual patients, these patterns and levels may change as the patient enters remission and relapse, reflecting changes in numbers and proportions of subclones.
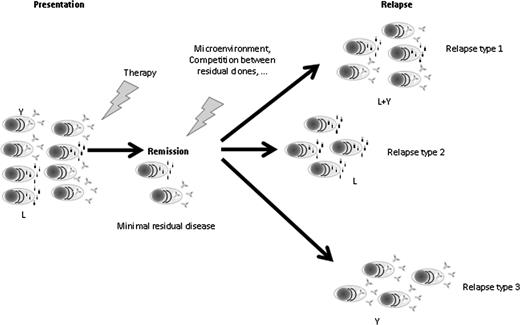
Overall, we describe that patients relapsing with increasing levels of FLC have a worse outcome from this time point than patients who relapse with increasing levels of only the whole paraprotein. Importantly, we describe for patients relapsing after presentation with an IgG or IgA paraprotein 10.4% relapse with FLC escape, 49.6% with an increase only in whole paraprotein, and 35.2% with an increase in both FLC and paraprotein levels. To explain these observations, we postulate that chemotherapy is differentially active against the clonal cells producing the intact paraprotein or the FLC-only secreting clone. In such a model, the type of relapse is a marker of a heterogeneous disease, in which different clones predominate at different time points (Figure 4). The better outcome from relapse for patients relapsing without increasing levels of FLC might reflect the presence of higher sensitivity to treatment in the clone characterized by whole paraprotein production only. This hypothesis is strengthened by the fact that having a relapse characterized only by an increase in paraprotein levels (PO) retained its prognostic value in a multivariate analysis that included treatment received at diagnosis (thalidomide vs nonthalidomide based), treatment pathway, and response achieved.
Model of Darwinian evolution in MM assessed by the type of paraprotein secreted: 1 clone is able to produce a complete antibody, whereas the other secretes only a FLC. Chemotherapy is differentially active against the different clones, as different as the impact of other evolutionary bottlenecks such as microenvironment or competition for the stem cell niche. The different selective pressures applied will determine which of the clone(s) will survive and give rise to the relapse. The different clonal composition at relapse will ultimately impact on the different sensitivities to subsequent treatments and therefore on survival. L, light chain only secreting plasma cells; Relapse type 1, relapse characterized by both free light chains and intact immunoglobulin secreting plasma cells; Relapse type 2, relapse characterized only by free light chains secreting plasma cells; Relapse type 3, relapse characterized only by intact immunoglobulin secreting plasma cells; Y, intact immunoglobulin secreting plasma cell.
Model of Darwinian evolution in MM assessed by the type of paraprotein secreted: 1 clone is able to produce a complete antibody, whereas the other secretes only a FLC. Chemotherapy is differentially active against the different clones, as different as the impact of other evolutionary bottlenecks such as microenvironment or competition for the stem cell niche. The different selective pressures applied will determine which of the clone(s) will survive and give rise to the relapse. The different clonal composition at relapse will ultimately impact on the different sensitivities to subsequent treatments and therefore on survival. L, light chain only secreting plasma cells; Relapse type 1, relapse characterized by both free light chains and intact immunoglobulin secreting plasma cells; Relapse type 2, relapse characterized only by free light chains secreting plasma cells; Relapse type 3, relapse characterized only by intact immunoglobulin secreting plasma cells; Y, intact immunoglobulin secreting plasma cell.
A longer survival from first relapse can also be related to the salvage treatment received. Data on treatment at relapse were available in less than one-third of the patients (157 of 520) and for this reason were not included in the analysis. However, the percentage of patients receiving a proteasome inhibitor was similar in the 3 groups identified at relapse (33%-34%). Only 1 patient was treated with lenalidomide. The proportions of patients receiving thalidomide at relapse were similar. Even if we cannot exclude an effect of treatment on survival after relapse, from available data this does not seem to be in contrast with our hypothesis that clones characterized by whole paraprotein production have a higher sensitivity to treatment.
Kumar et al reported higher median values of FLC ratios among patients with high-risk IgH translocation.27 However, they also found that the negative prognostic effect of very abnormal FLC levels was independent of the translocation abnormality [t(4;14) and t(14;16)].27 In our data set, IgH translocations were only available in 277 patients (53.1% of relapsed population) and, therefore, fluorescence in situ hybridization data were not included in the multivariate analysis. It is, however, worth noting that, in contrast to Kumar and colleagues, we did not find any significant difference between the median values at presentation and relapse of involved FLC or of FLC ratio in patients harboring high-risk IgH translocation (t(4;14,); t(14;16): t(14;20)) vs patients lacking these cytogenetic abnormalities.
The results presented here provide evidence to support the idea that intraclonal heterogeneity and clonal evolution are a general feature associated with disease progression and treatment resistance in myeloma. Further work needs to be carried out to allow us to precisely understand the underlying mechanism of unbalanced FLC excretion and how it affects survival, but in this study it acts as a marker of a clone with more aggressive clinical behavior. These results also illustrate the importance of disease monitoring using FLC analysis to ensure that FLC relapse is not missed.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by programme funding from Myeloma United Kingdom, the National Institute for Health Research Biomedical Research Centre at the Royal Marsden Hospital, the National Cancer Research Institute Haemato-Oncology subgroup.
F.E.D. is a Cancer Research United Kingdom Senior Clinical Fellow.
Authorship
Contribution: A.B., M.T.D., and G.J.M. designed and carried out research, analyzed data, and wrote the paper; G.J.M., J.A.C., and G.H.J. were chief investigators of the MRC Myeloma IX trial; H.G. and J.P.C. analyzed the data and contributed to the writing of the paper; M.F.K. and C.P. contributed to the writing of the paper; M.T.D. and W.M.G. collected the data; F.E.D., M.C., and L.M. provided important intellectual input and contributed to the writing of the paper; R.G.O. and F.E.D. provided patient samples; and all authors had access to, commented on, and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mark T. Drayson, Clinical Immunology Service, University of Birmingham Medical School, Birmingham, United Kingdom; e-mail: m.t.drayson@bham.ac.uk.
References
Author notes
M.T.D. and G.J.M. are joint senior authors.