Key Points
An in vitro system for the engulfment of pyrenocytes was established using erythroblastic islands.
MerTK, a receptor kinase, was essential for the engulfment of pyrenocytes by the central macrophages at erythroblastic islands.
Abstract
Definitive erythropoiesis takes place at erythroblastic islands, where erythroblasts proliferate and differentiate in association with central macrophages. At the final stage of erythropoiesis, pyrenocytes (nuclei surrounded by plasma membranes) are excluded from erythroblasts, expose phosphatidylserine (PtdSer), and are engulfed by the macrophages in a PtdSer-dependent manner. However, the molecular mechanism(s) involved in the engulfment of pyrenocytes are incompletely understood. Here, we constructed an in vitro assay system for the enucleation and engulfment of pyrenocytes using a methylcellulose-based culture. As reported previously, erythroblasts were bound to macrophages via interactions between integrin-α4β1 on erythroblasts and Vcam1 on macrophages. After enucleation, the resulting pyrenocytes exhibited a reduced affinity for Vcam1 that correlated with the presence of inactive integrin-α4β1 complexes. The pyrenocytes were then engulfed by the macrophages via a MerTK-protein S-dependent mechanism. Protein S appeared to function as a bridge between the pyrenocytes and macrophages by binding to PtdSer on the pyrenocytes and MerTK on the macrophages. Normally, NIH3T3 cells do not engulf pyrenocytes, but when they were transformed with MerTK, they efficiently engulfed pyrenocytes in the presence of protein S. These results suggest that macrophages use similar mechanisms to engulf both pyrenocytes and apoptotic cells.
Introduction
In mammals, red blood cells produced by definitive erythropoiesis lack nuclei due to enucleation. At the final stage of differentiation, nuclei are localized to 1 side of the cells and are extruded,1,2 giving rise to reticulocytes, which are released into the circulation, and pyrenocytes, which are the expelled nuclei surrounded by a plasma membrane. This process occurs on erythroblastic islands in the fetal liver and bone marrow.3 At the center of each island is a macrophage, which binds numerous erythroblasts during erythropoiesis. Pyrenocytes are engulfed by the central macrophages, thus preventing the release of nuclear contents, which can activate immune system.4
Every day, 2 × 1011 red blood cells are produced in a human adult, and the same number of pyrenocytes are engulfed and degraded. We previously showed that phosphatidylserine (PtdSer) is exposed on the plasma membrane of pyrenocytes as an “eat me” signal immediately after the separation of a pyrenocyte and its reticulocyte.1 PtdSer on apoptotic cells is the best characterized “eat me” signal, and factors that recognize PtdSer have been identified.4,5 For example, soluble proteins, such as MFG-E8, protein S, and Gas6, serve as bridges between apoptotic cells and macrophages, and stimulate the engulfment through their receptors, integrin-αvβ3 (for MFG-E8) or TAM kinase family members (for protein S and Gas6).6,7 However, it is currently unclear which molecules on the central macrophages are involved in recognizing PtdSer on pyrenocytes, leading to their engulfment.
The engulfment of apoptotic cells is divided into binding/tethering and uptake/tickling phases.8 The adhesion of erythroblasts and excluded pyrenocytes to central macrophages may also be important for the engulfment of pyrenocytes. Several adhesion molecules have been proposed for the attachment of erythroblasts to central macrophages.3 Among them, integrin-α4β1 on erythroblasts and Vcam1 on central macrophages are strong candidates because antibodies against these molecules disrupt erythroblastic islands.9 Moreover, integrin-α4β1 is reported to be enriched in pyrenocytes and excluded from reticulocytes,10 supporting the notion that reticulocytes are released into circulation, and that the integrin-α4β1–Vcam1 interaction promotes the engulfment of pyrenocytes.11 However, the direct binding of pyrenocytes to the central macrophages via Vcam1 has not been demonstrated.
Here, we purified erythroblastic islands from the spleens of phenylhydrazine (PHZ)-treated mice, and we constructed an in vitro culture system that recapitulated erythroblast enucleation and pyrenocyte engulfment. Using this system, we showed that the binding of erythroblasts to central macrophages was mediated by the interaction between Vcam1 and integrin-α4β1, but that pyrenocytes showed a reduced affinity for the macrophages, which correlated with the expression of inactive integrin-α4β1 on the surface of the pyrenocytes. The engulfment of pyrenocytes occurred rarely when erythroblastic islands were prepared from MerTK−/− mice, or when protein S was omitted from the culture medium, indicating that the central macrophage engulfment of pyrenocytes was protein S- and MerTK-dependent. In fact, mouse NIH3T3 cells expressing MerTK efficiently engulfed pyrenocytes in the presence of protein S. These results suggest that macrophages use similar mechanisms for engulfing pyrenocytes and apoptotic cells.
Methods
Mice, cell lines, recombinant proteins, reagents, and antibodies
C57BL/6 and MerTK−/−12 mice were from Japan SLC and Jackson Laboratory, respectively. Mice were housed in a specific pathogen-free facility at Kyoto University Graduate School of Medicine. All animal experiments were performed in accordance with protocols approved by the Animal Care and Use Committee at Kyoto University Graduate School of Medicine.
Mouse BaF3 was maintained in RPMI 1640 with 10% fetal calf serum (FCS) and 45 units/mL mouse IL-3, as described.13 Mouse NIH 3T3 and human 293T were grown in Dulbecco’s modified Eagle medium (DMEM)-10% FCS. PlatE14 was cultured in Dulbecco’s modified Eagle medium 10% FCS, 10 μg/mL blasticidin, and 1 μg/mL puromycin. Human erythropoietin (hEPO), transferrin, and protein S were from Chugai Pharmaceutical, Sigma, and Enzyme Research Laboratories, respectively. CellTracker Green (5-chloromethylfluorescein diacetate), pHrodo succinimidyl ester (pHrodo), SYTO16, SytoxBlue, and Alexa488-phalloidin were from Life Technologies. PHZ, fluorescein isothiocyanate–labeled peptide, 4-((n′-2-methylphenyl)ureido)-phenylacetyl-L-leucyl-L-aspartyl-L-valyl-L-prolyl-L-alanyl-L-alanyl-L-lysine (LDV-FITC), and Draq5 were from Nacalai Tesque, TOCRIS, and Biostatus, respectively. The pMXs retrovirus vector15 was from T. Kitamura (Institute of Medical Science, University of Tokyo). CSII-EF lentivirus vector, pCAG-HIVgp, pRSV-Rev, and pENV-IRES-puro were from H. Miyoshi (Riken Bioresource Center).
Hamster anti-F4/80 (clone 6-16A), a marker for mouse monocytes and tissue macrophages,16 was established in our laboratory and biotinylated. Phycoerythrin (PE)-rat anti-mouse Vcam1 (clone 429), PE-rat anti-mouse integrin-α4 (clone R1-2), PE-hamster anti-mouse integrin-β1 (clone HMβ1-1), allophycocyanin (APC) or PE-rat anti-mouse Ter119, and rat IgG2a-κ (clone RTK2758) were from BioLegend. APC-streptavidin, APC-anti-hamster IgG, and biotinylated goat anti-mouse MerTK were from BD Biosciences, Jackson Immunoresearch Laboratories, and R&D Systems, respectively.
Transformation
Vcam1 complementary DNA (cDNA) (GenBank: NM_011693) was prepared by reverse transcription-polymerase chain reaction from mouse spleen macrophages using primers (5′-ATATTTAATTAAGAGACTTGAAATGCCTGTGA-3′ and 5′-ATATGAATTCCACTTTGGATTTCTGTGCCT-3′), flag-tagged at C-terminus, and inserted into pMXs. To establish BaF3/Vcam1, Plat-E was transfected with pMXs carrying Vcam1 cDNA. Viruses were concentrated by centrifugation at 6,000 × g for 16 hours, and used to infect BaF3 in the presence of 10 μg/mL Polybrene. Transformants were selected by 800 μg/mL G-418. To transform NIH3T3, 293T was transfected with CSII-EF vector carrying MerTK cDNA and lentivirus-packaging constructs pCAG-HIVgp, pRSV-Rev, and pENV-IRES-puro. Viruses were concentrated (see above), and used to infect NIH3T3. Transformants were sorted with an anti-MerTK using a fluorescence-activated cell sorter (FACS)Aria II.
Analysis of spleen-derived cells and erythroblastic islands
C57/BL6 mice (8-20 weeks) were injected intraperitoneally 4 to 6 times every 2 days with PHZ at 40 mg/kg. For FACS analysis, spleens were minced, sheared with an 18-gauge syringe, and filtered through nylon mesh. Cells were washed with phosphate-buffered saline (PBS), incubated on ice for 30 minutes with 1 μg/mL APC-anti-Ter119, 200 nM SYTO16, and 200 nM SytoxBlue, and were analyzed on an FACSCanto II.
Erythroblastic islands were prepared from spleens, as previously described.17 In brief, spleens from PHZ-injected mice were minced and digested with 0.075% collagenase D and 0.004% DNase I in RPMI 1640 for 1 hour at 37°C on a shaker, passed through nylon mesh, and layered over RPMI 30% FCS. After 1 hour at room temperature (RT), the sedimented cells were washed twice with RPMI by centrifugation at 100 × g for 10 minutes, and allowed to adhere for 90 minutes at 37°C to 6 cm dishes in DMEM 10% FCS. For morphological analysis, the erythroblastic islands were fixed with formalin, permeabilized with PBS containing 0.1% bovine serum albumin (BSA) and 0.1% Triton-X, stained with 4 U/mL Alexa488-phalloidin, and 1 μg/mL 4′,6 diamidino-2-phenylindole (DAPI), and observed with FV1000D confocal microscopy (Olympus). To induce erythroblast differentiation, the islands were incubated overnight in Iscove modified Dulbecco medium (IMDM) 15% FCS, 3 U/mL hEPO, and 200 μg/mL transferrin. Cells were dispersed in PBS containing 1 mM EDTA, stained with 200 nM SYTO16 and 200 nM SytoxBlue, and analyzed with an FACSCanto II. For time-lapse analysis, erythroblastic islands on 3.5-cm glass dishes (Iwaki) were cultured in IMDM 15% FCS, 3 U/mL hEPO, and 200 μg/mL transferrin. Differential interference contrast images were obtained every 86 seconds using an FV1000D microscope.
Binding of erythroblasts to central macrophages
The central macrophages were identified as the cells that emit autofluorescence.18 In brief, cells in the erythroblastic islands were suspended in PBS containing 0.1% BSA and 1 mM EDTA (PBS/BSA/EDTA), stained with 200 nM SytoxBlue, and SytoxBluelow population was regarded as macrophages. In some cases, cells in the erythroblastic islands were incubated on ice for 30 minutes with 1 μg/mL biotinylated anti-F4/80 or anti-MerTK, or 1 μg/mL PE-anti-Vcam1, followed by incubation with 1 μg/mL APC-streptavidin and 200 nM SytoxBlue.
To assay the binding of erythroblasts to macrophages, cells from the erythroblastic islands were incubated with 1 μg/mL PE-anti-Ter119 in PBS/BSA/EDTA. Cells were then incubated for 30 minutes at RT in 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) buffer (pH 7.4) containing 150 mM NaCl (HEPES-buffered saline [HBS]) with 0.1% BSA and 1 mM EDTA (HBS/BSA/EDTA) or with 0.1% BSA, 1.8 mM CaCl2, and 0.8 mM MgCl2 (HBS/BSA/CaMg), stained with 200 nM SytoxBlue, and analyzed on an FACSCanto II. The Ter119 positive fraction in SytoxBluelow population was regarded as the macrophages carrying erythroid cells.
Preparation of erythroblasts, reticulocytes, and pyrenocytes
Spleen cells from PHZ-injected mice were incubated in DMEM 10% FCS, 3 U/mL hEPO, and 200 μg/mL transferrin for 5 hours at 37°C, stained with 200 nM SYTO16 and 200 nM SytoxBlue, and analyzed by FACS. The SYTO16+/Forward Scatter (FSC)high/ SytoxBlue−, SYTO16−/FSClow/SytoxBlue−, and SYTO16+/FSClow/SytoxBlue− cells represented erythroblasts, reticulocytes, and pyrenocytes, respectively.
To label erythroid cells with CellTracker, spleen cells from PHZ-treated mice were incubated with 10 μM CellTracker Green in DMEM at 37°C for 30 minutes, stained with 5 μM Draq5 and 200 nM SytoxBlue, and sorted into erythroblasts (Draq5+/FSChigh/SytoxBlue−), reticulocytes (Draq5−/FSClow/SytoxBlue−), and pyrenocytes (Draq5+/FSClow/SytoxBlue−) using an FACSAria II. To prepare pHrodo-labeled pyrenocytes, spleen cells were incubated at RT for 30 minutes with 0.2 μg/mL pHrodo in PBS, washed with PBS containing 10% FCS, stained with 5 μM Draq5 and 200 nM SytoxBlue. The Draq5+/FSClow/SytoxBlue- pyrenocytes were sorted into PBS containing 0.5% BSA and 0.25% globulin.
Binding and engulfment assays
To assay the binding of erythroblasts, reticulocytes, and pyrenocytes to BaF3, 2 × 104 BaF3 cells were incubated with 2 × 105 CellTracker-labeled erythroid cells in 200 μL of HBS/BSA/EDTA or HBS/BSA/CaMg for 30 minutes at RT, and analyzed by FACS. To assay the engulfment of pyrenocytes by NIH3T3, 2 × 104 NIH3T3 cells in 48-well plates were incubated with 2 × 105 pHrodo-pyrenocytes in DMEM at 37°C for 2 hours in the presence of 1 μg/mL protein S. The cells were trypsinized, suspended in N-Cyclohexyl-2-aminoethanesulfonic acid (CHES)-NaOH buffer (pH 9), and analyzed on an FACSAria II. In some cases, NIH3T3 plated on Lab-Tek II chamber cover glasses were incubated with pHrodo-pyrenocytes, exposed to CHES-NaOH buffer (pH 9), and observed with fluorescence microscopy (BioREVO BZ-9000; Keyence).
Pyrenocyte engulfment
Erythroblastic islands were cultured overnight in IMDM containing 1% methylcellulose, 3 U/mL hEPO, and 200 μg/mL transferrin, in the presence of FCS. The cells were washed with PBS, and gently trypsinized to remove erythroblasts. Macrophages were detached from the plates by treating with trypsin, suspended in PBS containing 2% FCS and 1 mM EDTA, fixed with 1% paraformaldehyde in PBS for 10 minutes, and permeabilized with 70% ethanol at −20°C for more than 3 hours. The cells were incubated in PBS containing 100 μg/mL RNase A for 30 minutes at RT, stained with 50 μg/mL propidium iodide (PI) for 30 minutes at 4°C, and analyzed on an FACSCanto II. For microscopic observation, the stained cells were transferred to a glass-bottom 96-well plate (Asahi Techno Glass) and observed by fluorescence microscopy (BioREVO).
Binding of FITC-conjugated LDV-peptides
The activation of integrin-α4β1 was determined by LDV-FITC binding.19 In brief, cells from PHZ-treated mouse spleen were incubated in DMEM 10% FCS, 3 U/mL hEPO, and 200 μg/mL transferrin for 5 hours at 37°C. Three ×106 cells were then incubated with 10 nM LDV-FITC in HBS/BSA for 30 minutes at RT, stained with 5 μM Draq5 and 200 nM SytoxBlue, and analyzed with an FACSCanto II.
Electron microscopy
Erythroblastic islands were cultured overnight in IMDM containing 1% methylcellulose, 3 U/mL hEPO, and 200 μg/mL transferrin, and macrophages were prepared as described (see above). Cells were fixed in PBS containing 4% paraformaldehyde and 2% glutaraldehyde at 4°C overnight, embedded in 2% agar, and postfixed at 4°C for 2 hours with 1% OsO4. Samples were successively dehydrated in serially diluted ethanol (50%, 60%, 70%, 80%, 90%, and 99% ethanol) for 10 minutes, respectively, followed by 30 minutes incubation in 100% ethanol twice. The samples were incubated in propylene oxide for 30 minutes twice, in a 1:1 mixture of propylene oxide and epoxide (Luveak 812; Nacalai Tesque) for 1.5 hours, in a 1:3 mixture of propylene oxide and epoxide for 1.5 hours, in epoxide for 12 hours, and embedded in epoxide at 60°C for 3 days. Ultrathin sections (60-80 nm) were cut with an ultramicrotome EM UC6 (Leica), stained with uranyl acetate and lead citrate, and observed with an electron microscope H-7650 (Hitachi).
Results
Preparation of erythroblastic islands
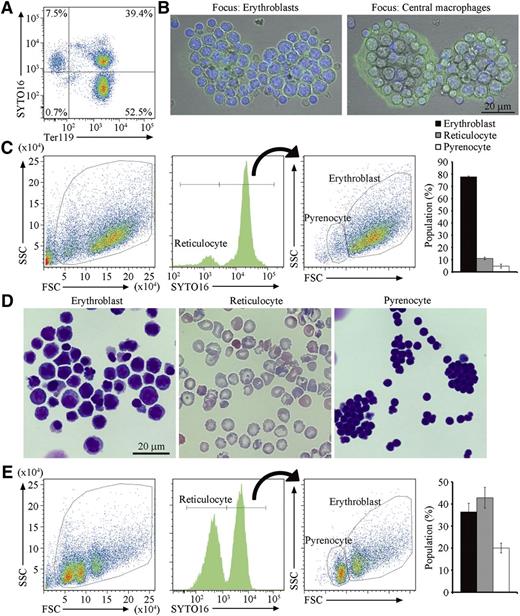
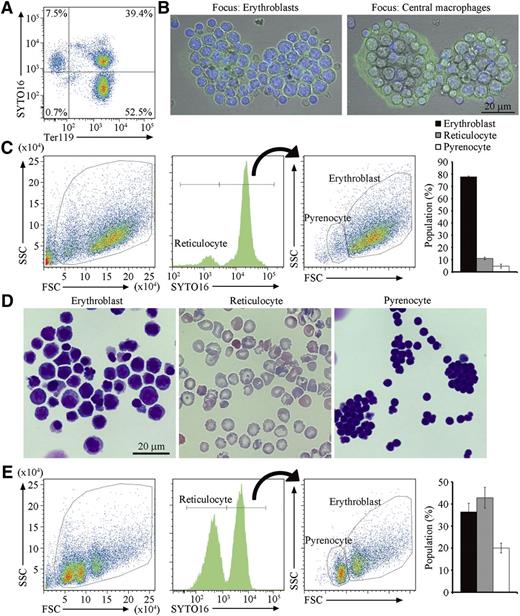
To obtain a large number of erythroblastic islands containing developmentally synchronized erythroblasts, extramedullary hematopoiesis in the spleens was induced by treating mice with 4 to 6 doses of PHZ at 40 mg/kg. After this treatment, the mice were anemic, and the size of their spleens were 5 times larger than that of untreated controls. At this stage, more than 90% of cells from the spleen expressed Ter119, a marker for the cells at the late stage of murine erythroid lineage,20 and consisted of erythroblasts and reticulocytes, distinguished by staining with SYTO16 or Draq5, cell-permeable DNA-staining dyes21,22 (Figure 1A).
Erythroblastic islands from the spleen of PHZ-treated mice. (A) Cells from PHZ-injected C57BL/6J mouse spleen were stained with SYTO16 and APC-labeled anti-Ter119, and analyzed on an FACSCanto II. The staining profiles for SYTO16 and Ter119 in SytoxBlue-negative cells are shown. (B) Erythroblastic islands were stained with DAPI and Alexa488-conjugated phalloidin, and observed by FV1000D confocal microscopy with an objective lens magnification of ×60. Merged images of cells stained with DAPI (blue) and phalloidin (green) are shown. The left and right panels show images focusing on erythroblasts and central macrophages, respectively. Scale bar, 20 μm. (C) Cells in the erythroblastic islands were stained with SYTO16 and analyzed by FACS. The FSC-SSC (left panel), and the SYTO16-staining (middle panel) profiles of the SytoxBlue-negative population are shown. The SYTO16-negative population represents reticulocytes. The FSC-SSC profile of the SYTO16-positive cell population is shown on the right, in which the FSClow and FSChigh populations represent pyrenocytes and erythroblasts, respectively. The percentage of cells in each fraction was calculated. Experiments were performed 3 times with different mice, and the average values are shown with standard deviation (vertical bars). (D) The indicated sorted cell population was cytospun and stained with Wright-Giemsa, and observed by BioRevo fluorescence microscope with objective lens magnification of ×100. (E) The erythroblastic islands were cultured overnight, and the cells in the entire culture were stained with SYTO16. The FSC-SSC- and SYTO16-staining profiles, and the percentage of each cell population are shown as described above (C).
Erythroblastic islands from the spleen of PHZ-treated mice. (A) Cells from PHZ-injected C57BL/6J mouse spleen were stained with SYTO16 and APC-labeled anti-Ter119, and analyzed on an FACSCanto II. The staining profiles for SYTO16 and Ter119 in SytoxBlue-negative cells are shown. (B) Erythroblastic islands were stained with DAPI and Alexa488-conjugated phalloidin, and observed by FV1000D confocal microscopy with an objective lens magnification of ×60. Merged images of cells stained with DAPI (blue) and phalloidin (green) are shown. The left and right panels show images focusing on erythroblasts and central macrophages, respectively. Scale bar, 20 μm. (C) Cells in the erythroblastic islands were stained with SYTO16 and analyzed by FACS. The FSC-SSC (left panel), and the SYTO16-staining (middle panel) profiles of the SytoxBlue-negative population are shown. The SYTO16-negative population represents reticulocytes. The FSC-SSC profile of the SYTO16-positive cell population is shown on the right, in which the FSClow and FSChigh populations represent pyrenocytes and erythroblasts, respectively. The percentage of cells in each fraction was calculated. Experiments were performed 3 times with different mice, and the average values are shown with standard deviation (vertical bars). (D) The indicated sorted cell population was cytospun and stained with Wright-Giemsa, and observed by BioRevo fluorescence microscope with objective lens magnification of ×100. (E) The erythroblastic islands were cultured overnight, and the cells in the entire culture were stained with SYTO16. The FSC-SSC- and SYTO16-staining profiles, and the percentage of each cell population are shown as described above (C).
Erythroblastic islands were prepared from PHZ-treated spleens according to the method of Crocker and Gordon,17 in which cells are enzymatically dispersed, and erythroblastic islands are purified by sedimentation on a layer of 30% FCS without centrifugation. Staining of the erythroblastic islands with phalloidin and DAPI indicated that a macrophage with extended lamellipodia was present in the center of the erythroblastic islands adhered to the glass dishes, and that 20 to 30 erythroblasts were associated with each macrophage (Figure 1B). Cells in the erythroblastic islands were then stained with SYTO16 and analyzed by flow cytometry (Figure 1C). The SYTO16+ cells consisted of 2 populations: erythroblasts with high FSC, and pyrenocytes with low FSC, whereas SYTO16− cells with low FSC were reticulocytes (Figure 1D). Approximately 80% of the cells in the erythroblastic islands were erythroblasts (Figure 1C). When the erythroblastic islands were cultured overnight in the presence of hEPO and transferrin, the number of erythroblasts in the culture decreased, whereas the proportion of reticulocytes increased from 10% to 42% (Figure 1E). These results indicated that erythroblasts on the central macrophages were capable of undergoing differentiation. The disparity of the pyrenocyte (30%) and reticulocyte (42%) populations was most likely due to macrophage engulfment of the pyrenocytes and/or fragile nature of pyrenocytes that quickly became SytoxBlue positive.
Binding of erythroblasts to Vcam1-expressing cells
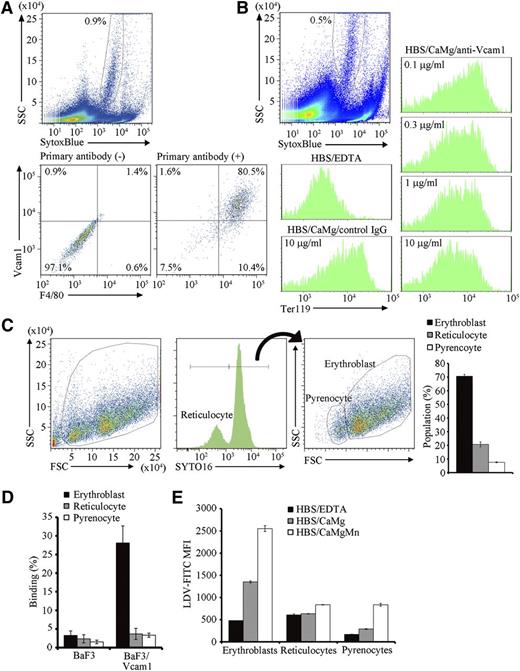
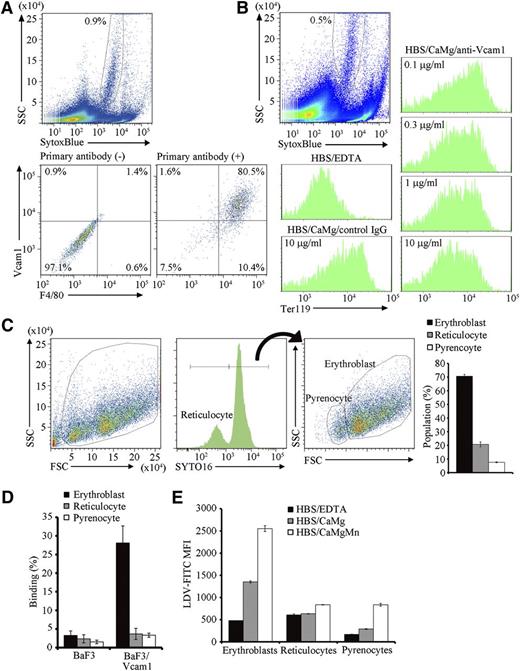
Red pulp macrophages, or macrophages in the erythroblastic islands, are heterogenous and emit autofluorescence.18 Approximately 0.9% cells from the erythroblastic islands carried strong autofluorescence with a heterogenous side scatter (SSC) profile and expressed F4/80 and Vcam1 (Figure 2A), indicating that this population are macrophages in erythroblastic islands. As previously reported,17 when cells in freshly isolated erythroblastic islands were dispersed in PBS/BSA/EDTA, and then incubated in buffer containing Ca2+ and Mg2+, they reassociated (Figure 2B). The reassociation was significantly inhibited by the presence of anti-Vcam1 antibody (Figure 2B). These results confirmed that erythroblasts bind to the macrophages via Vcam1,9 although we cannot rule out the possible involvement of other molecules such as ICAM-4/Integrin α5 in this process.23,24 When the erythroblastic islands were incubated overnight, a significant number of reticulocytes and pyrenocytes were produced (Figure 1E), but only erythroblasts were found to associate with the central macrophages (Figure 2C), suggesting that reticulocytes and pyrenocytes have little affinity for the macrophages.
Binding of erythroblasts to Vcam1-expressing macrophages. (A) Cells in the erythroblastic islands were suspended in PBS/BSA/EDTA, and stained with biotinylated hamster anti-F4/80, followed by staining with SytoxBlue, APC-labeled anti-hamster IgG, and PE-labeled anti-Vcam1, and analyzed on an FACSCanto II. Macrophages emit autofluorescence in the Pacific blue channel (blue laser; excitation 405, emission 450/50).18 Thus, the SytoxBluelow population (upper panel) was analyzed for F4/80 and Vcam1 expression with or without primary antibodies (lower panels). (B) Vcam1-dependent binding of erythroblasts to central macrophages. Cells in erythroblastic islands were suspended in PBS/BSA/EDTA and stained with PE-anti-Ter119. The cells were then incubated in HBS/BSA/EDTA, or in HBS/BSA/CaMg in the presence of 10 μg/mL control IgG or in the indicated amount of anti-Vcam1 at RT for 30 minutes, and analyzed on an FACSCanto II. The Ter119-staining profiles associated with the SytoxBluelow macrophage population (upper panel) are shown. (C) The erythroblastic islands were cultured overnight, washed with HBS/CaMg, and the cells in the islands were stained with SYTO16. The FSC-SSC- and SYTO16-staining profiles, and the percentage of each cell population are shown. (D) BaF3 and BaF3/Vcam1 cells were incubated with CellTracker-labeled erythroblasts, reticulocytes, or pyrenocytes at RT for 30 minutes in HBS/BSA/CaMg, and analyzed on an FACSCanto II (supplemental Figure 2, available on the Blood Web site). The numbers indicate the percentage of CellTracker-positive cells. Experiments were performed in triplicate, and the average values are shown with standard deviation. (E) Cells from the PHZ-treated mouse spleen were incubated for 5 hours at 37°C. The cells were then incubated with 10 nM LDV-FITC for 30 minutes at RT in HBS/BSA/EDTA, HBS/BSA/CaMg, or HBS/BSA/CaMgMn, stained with Draq5 and SytoxBlue, and analyzed on an FACSCanto II. The FITC mean fluorescence intensity (MFI) in the SytoxBlue− fraction was determined for the Draq+FSChigh (erythroblasts), Draq+FSClow (pyrenocytes), and Draq− (reticulocytes) populations. Experiments were performed in triplicate, and the average values were plotted with standard deviation (vertical bars). All experiments in Figure 2 were carried out several times with erythroblastic islands prepared from different mice, and representative results are shown.
Binding of erythroblasts to Vcam1-expressing macrophages. (A) Cells in the erythroblastic islands were suspended in PBS/BSA/EDTA, and stained with biotinylated hamster anti-F4/80, followed by staining with SytoxBlue, APC-labeled anti-hamster IgG, and PE-labeled anti-Vcam1, and analyzed on an FACSCanto II. Macrophages emit autofluorescence in the Pacific blue channel (blue laser; excitation 405, emission 450/50).18 Thus, the SytoxBluelow population (upper panel) was analyzed for F4/80 and Vcam1 expression with or without primary antibodies (lower panels). (B) Vcam1-dependent binding of erythroblasts to central macrophages. Cells in erythroblastic islands were suspended in PBS/BSA/EDTA and stained with PE-anti-Ter119. The cells were then incubated in HBS/BSA/EDTA, or in HBS/BSA/CaMg in the presence of 10 μg/mL control IgG or in the indicated amount of anti-Vcam1 at RT for 30 minutes, and analyzed on an FACSCanto II. The Ter119-staining profiles associated with the SytoxBluelow macrophage population (upper panel) are shown. (C) The erythroblastic islands were cultured overnight, washed with HBS/CaMg, and the cells in the islands were stained with SYTO16. The FSC-SSC- and SYTO16-staining profiles, and the percentage of each cell population are shown. (D) BaF3 and BaF3/Vcam1 cells were incubated with CellTracker-labeled erythroblasts, reticulocytes, or pyrenocytes at RT for 30 minutes in HBS/BSA/CaMg, and analyzed on an FACSCanto II (supplemental Figure 2, available on the Blood Web site). The numbers indicate the percentage of CellTracker-positive cells. Experiments were performed in triplicate, and the average values are shown with standard deviation. (E) Cells from the PHZ-treated mouse spleen were incubated for 5 hours at 37°C. The cells were then incubated with 10 nM LDV-FITC for 30 minutes at RT in HBS/BSA/EDTA, HBS/BSA/CaMg, or HBS/BSA/CaMgMn, stained with Draq5 and SytoxBlue, and analyzed on an FACSCanto II. The FITC mean fluorescence intensity (MFI) in the SytoxBlue− fraction was determined for the Draq+FSChigh (erythroblasts), Draq+FSClow (pyrenocytes), and Draq− (reticulocytes) populations. Experiments were performed in triplicate, and the average values were plotted with standard deviation (vertical bars). All experiments in Figure 2 were carried out several times with erythroblastic islands prepared from different mice, and representative results are shown.
To confirm this finding in a reconstituted system, BaF3 cells that did not express Vcam1 were transformed with Vcam1 (BaF3/Vcam1) (supplemental Figure 1), and incubated with CellTracker-labeled erythroblasts, reticulocytes, or pyrenocytes (supplemental Figure 2A). BaF3 with FSChigh-SSChigh did not bind erythroblasts, reticulocytes, or pyrenocytes (supplemental Figure 2B; Figure 2D). In contrast, approximately 30% of the BaF3/Vcam1 bound erythroblasts, with little binding observed to reticulocytes and pyrenocytes. Vcam1 is a ligand for VLA-4 (very late antigen-4, integrin-α4β1) and mediates cellular interactions.25 Integrin-α4β1 has several activation states, and the physiologically activated form can be detected by binding of LDV-FITC.19 As shown in Figure 2E, the erythroblasts bound LDV-FITC in buffer containing 1.8 mM Ca2+ and 0.8 mM Mg2+. On the other hand, very little LDV-FITC binding was observed with reticulocytes and pyrenocytes. Interestingly, a significant increase in the binding was observed with pyrenocytes in buffer containing 0.5 mM Mn2+, a condition that aberrantly activates integrin-α4β1.19 In fact, the expression of integrin-α4 and integrin-β1 on pyrenocytes was confirmed by staining with anti-integrin-α4 and -β1 (supplemental Figure 3).
Engulfment of pyrenocytes by central macrophages
To study the engulfment of pyrenocytes, we monitored the erythroblastic islands using time-lapse video. As shown in supplemental Video 1, erythroblasts frequently initiated enucleation, but pyrenocytes and reticulocytes were not separated, and the enucleating erythroblasts sometimes left the islands. This apparently contradicts the data obtained by FACS analysis, in which erythroblasts were found to undergo enucleation (Figure 1E). Reticulocytes and pyrenocytes dissociate from enucleating erythroblasts in response to weak physical stress.1 During FACS analysis, cells of the erythroblastic islands were suspended by pipetting, which may have dissociated pyrenocytes and reticulocytes from the enucleating erythroblasts.
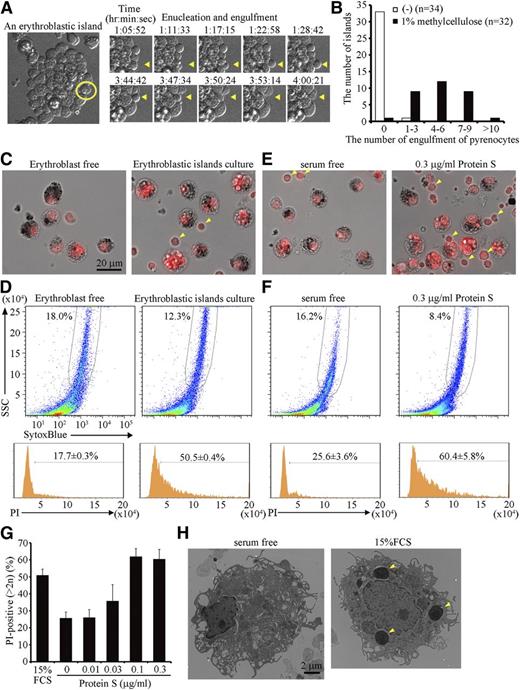
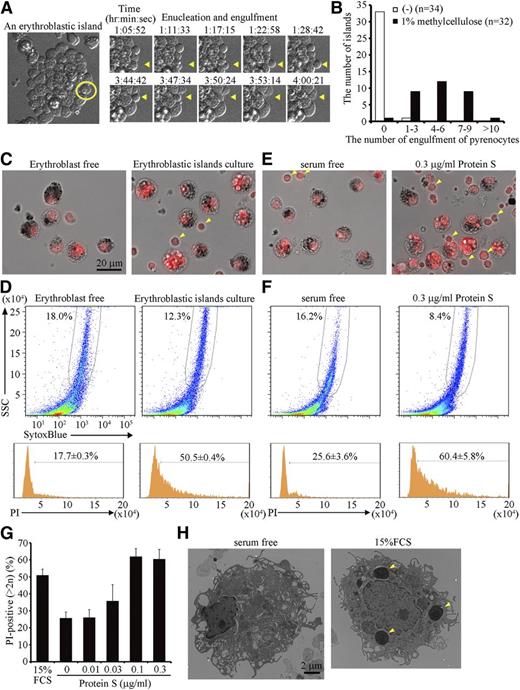
Time-lapse observation of the erythroblastic islands also indicated that very few pyrenocytes were engulfed by the central macrophages (supplemental Video 1). However, when the viscosity of the medium was increased with 1% methylcellulose, thus increasing the level of shear-stress to the islands, dissociation of pyrenocytes from reticulocytes, as well as engulfment of pyrenocytes occurred more frequently (Figure 3A; supplemental Video 2). More than half of the macrophages in the erythroblastic islands engulfed at least 5 pyrenocytes each within 14 hours in the presence of 1% methylcellulose (Figure 3B).
Engulfment of pyrenocytes in erythroblastic islands. (A-D) Erythroblastic islands were cultured overnight in IMDM containing 15% FCS, 3 U/mL hEPO, and 200 μg/mL transferrin in the presence or absence of 1% methylcellulose. (A) An erythroblastic island cultured in the presence of methylcellulose is observed by FV1000D microscope with an objective lens magnification of ×60, and is shown on the left. An erythroblast, in which enucleation and engulfment of the expelled pyrenocyte were followed every 86 seconds (right panels and supplemental Video 2), is circled in yellow. Yellow arrowheads in the right panels point to enucleation of the erythroblast and engulfment of its pyrenocyte. (B) Quantification of the number of pyrenocytes engulfed during a 14-hour incubation in the presence or absence of 1% methylcellulose. The number of erythroblastic islands examined in the cultures with and without methylcellulose was 32 and 34, respectively. (C-D) After an overnight culture in 1% methylcellulose, the erythroblastic islands were washed to remove erythroblasts and reticulocytes, stained with PI, and observed by BioRevo fluorescence microscopy with an objective lens magnification of ×40 (C), or subjected to FACS analysis (D). As a control, the erythroblastic islands were depleted of erythroblasts before the overnight culture. (C) macrophages carrying dark debris can be distinguished from erythroblasts (pointed by arrowheads) that are smaller than macrophages. Scale bar, 20 μm. (D) The PI-staining profiles for the SytoxBluelow macrophages are shown. The experiments were performed 3 times, and the percentage of cells containing extra PI-positive material is shown with standard deviation. (E-H) Erythroblastic islands were cultured overnight in the presence of 1% methylcellulose, with or without the indicated concentrations of protein S. After removing erythroblasts, the adherent cells were stained with PI and observed by fluorescence microscopy (E), or analyzed on an FACSCanto II (F), as described in Figure 3C-D. The experiments were performed 3 times, and the percentage of cells containing extra PI-positive material is shown with a standard deviation (vertical bars) (G). (H) The adherent cells in erythroblastic islands cultured in the absence or presence of 15% FCS were observed by electron transmission microscope with magnification of ×2500. Yellow arrowheads indicate the engulfed pyrenocytes.
Engulfment of pyrenocytes in erythroblastic islands. (A-D) Erythroblastic islands were cultured overnight in IMDM containing 15% FCS, 3 U/mL hEPO, and 200 μg/mL transferrin in the presence or absence of 1% methylcellulose. (A) An erythroblastic island cultured in the presence of methylcellulose is observed by FV1000D microscope with an objective lens magnification of ×60, and is shown on the left. An erythroblast, in which enucleation and engulfment of the expelled pyrenocyte were followed every 86 seconds (right panels and supplemental Video 2), is circled in yellow. Yellow arrowheads in the right panels point to enucleation of the erythroblast and engulfment of its pyrenocyte. (B) Quantification of the number of pyrenocytes engulfed during a 14-hour incubation in the presence or absence of 1% methylcellulose. The number of erythroblastic islands examined in the cultures with and without methylcellulose was 32 and 34, respectively. (C-D) After an overnight culture in 1% methylcellulose, the erythroblastic islands were washed to remove erythroblasts and reticulocytes, stained with PI, and observed by BioRevo fluorescence microscopy with an objective lens magnification of ×40 (C), or subjected to FACS analysis (D). As a control, the erythroblastic islands were depleted of erythroblasts before the overnight culture. (C) macrophages carrying dark debris can be distinguished from erythroblasts (pointed by arrowheads) that are smaller than macrophages. Scale bar, 20 μm. (D) The PI-staining profiles for the SytoxBluelow macrophages are shown. The experiments were performed 3 times, and the percentage of cells containing extra PI-positive material is shown with standard deviation. (E-H) Erythroblastic islands were cultured overnight in the presence of 1% methylcellulose, with or without the indicated concentrations of protein S. After removing erythroblasts, the adherent cells were stained with PI and observed by fluorescence microscopy (E), or analyzed on an FACSCanto II (F), as described in Figure 3C-D. The experiments were performed 3 times, and the percentage of cells containing extra PI-positive material is shown with a standard deviation (vertical bars) (G). (H) The adherent cells in erythroblastic islands cultured in the absence or presence of 15% FCS were observed by electron transmission microscope with magnification of ×2500. Yellow arrowheads indicate the engulfed pyrenocytes.
To quantify the pyrenocyte engulfment by macrophages, the erythroblasts were removed, and the macrophages were stained with PI. As shown in Figure 3C, the macrophages contained several PI-positive foci in addition to their own nuclei. These PI-positive foci were not observed if erythroblasts were removed before the islands were cultured. FACS analysis indicated that approximately 50% of the macrophages contained the extra PI-positive materials (Figure 3D). In contrast, less than 20% of the macrophages cultured without erythroblasts carried extra PI-positive material, which may have reflected the cells in S-phase.26 The engulfment of apoptotic cells by resident macrophages depends on fetal calf serum.27 Similarly, when serum was removed from the culture medium for erythroblastic islands, the accumulation of the extra PI-positive material was significantly reduced (Figure 3E-G). Observation of the macrophages by electron microscope confirmed that the engulfment of pyrenocytes in the presence of serum (Figure 3H). The number of pyrenocytes generated from the erythroblasts did not differ between the cultures with or without FCS (supplemental Figure 4), indicating that the engulfment, but not the enucleation process, required FCS.
Protein S and MerTK-dependent engulfment of pyrenocytes
It was previously reported that protein S in serum recognizes PtdSer and stimulates the phagocytosis of apoptotic cells.27 Similarly, we found that protein S dose-dependently enhanced the engulfment of pyrenocytes in erythroblastic islands in serum-free medium, and that 0.1 μg/mL protein S had a comparable effect to 15% FCS (Figure 3E-G).
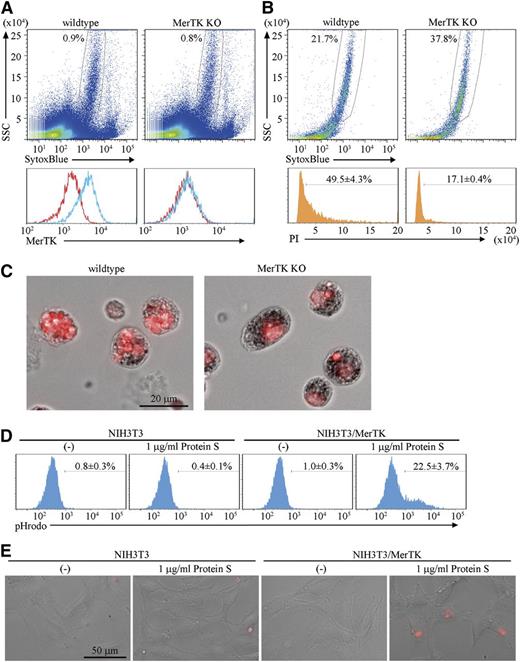
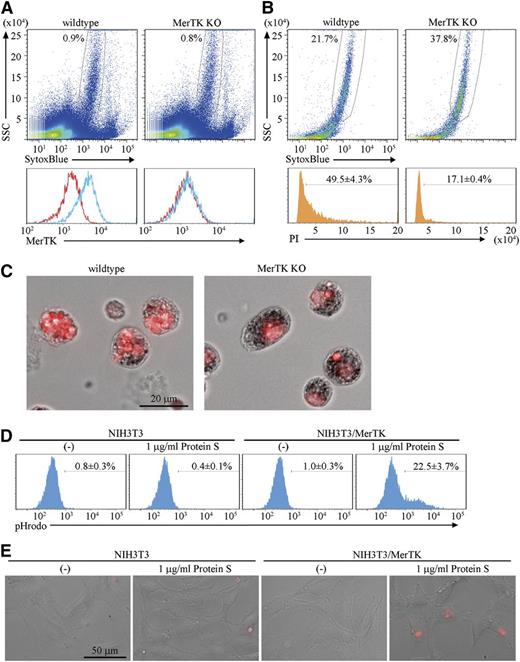
Protein S is a ligand for MerTK and functions as a bridge between apoptotic cells and macrophages.7 Because the macrophages in erythroblastic islands expressed MerTK (Figure 4A), the involvement of MerTK in the engulfment of pyrenocytes was examined using erythroblastic islands prepared from MerTK−/− mice. When the erythroblasts were cultured overnight, a similar number of pyrenocytes were produced from the wild-type and MerTK−/− erythroblasts (supplemental Figure 4). On the other hand, the PI-positive materials accumulating in the macrophages of erythroblastic islands were remarkably reduced by the lack of MerTK (Figure 4B-C), suggesting that MerTK is important for the engulfment of pyrenocytes by the central macrophages.
MerTK-mediated engulfment of pyrenocytes. (A) Cells of the erythroblastic islands prepared from wild-type or MerTK−/− mice were dispersed in PBS/BSA/EDTA, and analyzed by FACS for MerTK expression. The MerTK-staining profile in SytoxBluelow population (upper panel) with (blue) or without (red) primary antibody is shown. (B-C) Erythroblastic islands from wild-type and MerTK−/− mice were cultured overnight in medium containing 1% methylcellulose, washed to remove erythroblasts, stained with PI, and analyzed on an FACSCanto II. The PI-staining profile in SytoxBluelow population is shown. The experiments were performed 3 times, and the average percentage of cells containing extra PI-positive materials are shown with standard deviation (B). The PI-stained samples were observed by BioRevo fluorescence microscopy with objective lens’s magnification of 40 (C). (D) NIH3T3 MerTK transformants were incubated at 37°C for 2 hours with pHrodo-labeled pyrenocytes in serum-free medium with or without 1 μg/ml Protein S, and subjected to FACSAria II analysis. Experiments were performed three times, and the average percentages of pHrodo-positive cells are shown with standard deviation. (E). NIH3T3 and MerTK transformants were plated on Labo-Tek chamber cover glasses, incubated with pHrodo-labeled pyrenocytes in serum-free medium with or without protein S, and observed by BioRevo fluorescence microscopy with an objective lens magnification of ×40. Scale bar, 50 μm.
MerTK-mediated engulfment of pyrenocytes. (A) Cells of the erythroblastic islands prepared from wild-type or MerTK−/− mice were dispersed in PBS/BSA/EDTA, and analyzed by FACS for MerTK expression. The MerTK-staining profile in SytoxBluelow population (upper panel) with (blue) or without (red) primary antibody is shown. (B-C) Erythroblastic islands from wild-type and MerTK−/− mice were cultured overnight in medium containing 1% methylcellulose, washed to remove erythroblasts, stained with PI, and analyzed on an FACSCanto II. The PI-staining profile in SytoxBluelow population is shown. The experiments were performed 3 times, and the average percentage of cells containing extra PI-positive materials are shown with standard deviation (B). The PI-stained samples were observed by BioRevo fluorescence microscopy with objective lens’s magnification of 40 (C). (D) NIH3T3 MerTK transformants were incubated at 37°C for 2 hours with pHrodo-labeled pyrenocytes in serum-free medium with or without 1 μg/ml Protein S, and subjected to FACSAria II analysis. Experiments were performed three times, and the average percentages of pHrodo-positive cells are shown with standard deviation. (E). NIH3T3 and MerTK transformants were plated on Labo-Tek chamber cover glasses, incubated with pHrodo-labeled pyrenocytes in serum-free medium with or without protein S, and observed by BioRevo fluorescence microscopy with an objective lens magnification of ×40. Scale bar, 50 μm.
To examine whether the protein S–MerTK system is sufficient for promoting the engulfment of pyrenocytes, NIH3T3, which did not engulf pyrenocytes, was transformed with MerTK (supplemental Figure 5), and incubated with pHrodo-labeled pyrenocytes, which emit strong fluorescent signals in acidic lysosomes.13,28 As shown in Figure 4D-E and supplemental Figure 5, NIH3T3/MerTK, but not NIH3T3, engulfed pyrenocytes in the presence of 1 μg/mL protein S. Ten to 20% of the NIH3T3/MerTK carried 2 to 3 pHrodo-positive pyrenocytes after a 2- to 4-hour incubation with pyrenocytes.
Discussion
Red blood cells are generated at erythroblastic islands, where a single macrophage located at the center interacts with a number of differentiating erythroblasts. The production of red blood cells consists of 4 steps: (1) erythroblast binding to macrophages; (2) erythroblast maturation accompanied by enucleation; (3) separation into reticulocytes and pyrenocytes; and (4) pyrenocyte engulfment by macrophages and release of reticulocytes into the circulation. Although macrophages play a crucial role in definitive erythropoiesis,29,30 the molecular mechanism(s) of pyrenocyte engulfment have been elusive.3,31 In this report, we prepared erythroblastic islands from the spleen of mice induced to undergo hemophilic anemia. Culturing the islands in methylcellulose-containing medium strongly enhanced pyrenocyte enucleation and suggested that PtdSer-exposing pyrenocytes are engulfed by macrophages via a mechanism similar to that used for the engulfment of apoptotic cells, at least in the stress-induced erythropoiesis in the mouse spleen.
Erythroblasts bind to macrophages, and when they undergo maturation, reticulocytes leave the islands while pyrenocytes are engulfed by the macrophages.3 Thus, it was postulated that cell-adhesion molecules that are evenly distributed on the surface of erythroblasts are retained by pyrenocytes, but may be lost from the surface of reticulocytes.32 However, here we showed that while erythroblasts exhibited a high affinity for the central macrophages, both reticulocytes and pyrenocytes lost the affinity. As reported previously,9 the functionally active α4β1-integrin complex was present on erythroblasts, but its level was severely decreased on reticulocytes and pyrenocytes. The total level of α4β1-integrin, detected by LDV peptide in the presence of Mn2+, was also reduced in reticulocytes and pyrenocytes, suggesting that the expression of α4- and β1-integrins was downregulated during erythroblast maturation. In addition, pyrenocytes are devoid of adenosine triphosphate,1 which may also explain the low level of activated α4β1 in pyrenocytes because intracellular adenosine triphosphate is required for integrin-α4β1 to bind to Vcam1.33
Except for a few reports,34,35 erythroid enucleation is rarely observed when erythroid cells are cultured in vitro.36 A time-lapse observation of erythroblastic islands cultured in the absence of methylcellulose showed that erythroblasts underwent partial enucleation and left the islands without releasing pyrenocytes. The dissociation of reticulocytes from pyrenocytes was rarely observed. On the other hand, reticulocytes were frequently separated from pyrenocytes when the islands were cultured in the presence of methylcellulose. We previously showed that shear stress is required for pyrenocytes to dissociate from reticulocytes.1 Shear stress is determined by viscosity of the solution and the velocity gradient of flow.37 Plasma membrane of the macrophages in erythroblastic islands form dynamic ruffles, which likely generate fluid flow across the closely associated erythroblasts. The strength of the flow may be similar between the cultures with or without methylcellulose; however, the high viscosity generated by methylcellulose appears to provide shear stress to induce the dissociation of reticulocytes and pyrenocytes. Although the pyrenocytes exhibited a reduced affinity for macrophages, they were engulfed by the macrophages in the presence of methylcellulose, suggesting that the high viscosity generated by methylcellulose may have been sufficient to maintain the close proximity of pyrenocytes and macrophages. In bone marrow, erythroblastic islands are surrounded by matrix proteins such as fibronectins and laminins,3 which are likely to provide viscosity comparable to 1% methylcellulose.
As with apoptotic cells, pyrenocytes expose PtdSer,1 and are engulfed by macrophages in a PtdSer-dependent manner. Among several systems proposed for the engulfment of apoptotic cells, the MerTK/protein S system plays an essential role in the PtdSer-dependent engulfment of apoptotic cells.7 Similarly, a MerTK- deficiency blocked the engulfment of pyrenocytes by the central macrophages in vitro culture of erythroblastic islands (Figure 4B-C). In contrast, the bone marrow and spleens of MerTK-deficient mice were apparently normal (S.T., K.S., and S.N., unpublished observation),12 suggesting that a compensatory system operates in vivo, but not in vitro. Axl, another member of the TAM family was expressed in the macrophages of the erythroblastic islands (S.T., K.S., and S.N., unpublished results), while the concentration of Gas6, the ligand for Axl38 is low in the serum,39 which may explain this difference.
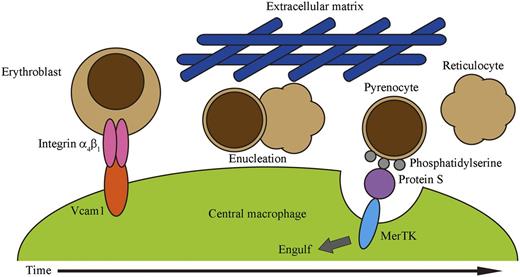
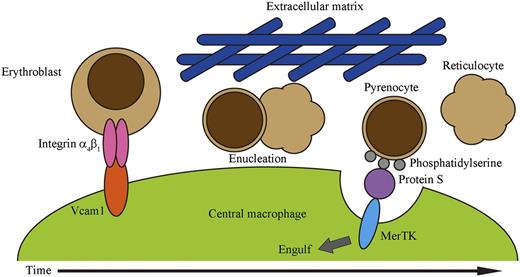
In summary, we propose the following model for erythropoiesis on erythroblastic islands (Figure 5). Erythroblasts bind to the central macrophage via the interaction between integrin-α4β1 on erythroblasts and Vcam1 expressed on the macrophage. Erythroblasts proliferate in association with the macrophages, and undergo maturation followed by enucleation, which is accompanied by downregulation of integrin-α4β1. The viscosity generated by matrix proteins surrounding erythroblastic islands provides shear stress required for the dissociation of pyrenocytes and reticulocytes. Reticulocytes leave the erythroblastic islands and enter the circulation, while the dissociated pyrenocytes expose PtdSer. The central macrophages then engulf pyrenocytes using a MerTK-dependent mechanism. Recently, systems have been developed to produce human red blood cells, in which erythroid precursor cells or embryonic stem cells are cultured in a step-wise fashion with several growth factors in the presence of stromal cells.40,41 Although these systems are successful in generating a large number of mature erythroid cells, most of the erythrocytes are not enucleated.36 We showed here that the presence of methylcellulose was sufficient to enhance the separation of reticulocytes from pyrenocytes, and that MerTK-expressing cells efficiently engulfed pyrenocytes. This information should be useful for developing improved systems for producing mature enucleated red blood cells from erythroid precursors.
A model for definitive erythropoiesis on erythroblastic islands. Erythroblasts bind to a central macrophage through the interactions of integrin-α4β1 on erythroblasts and Vcam1 on macrophages. Erythroblasts then undergo enucleation, in which the separation of pyrenocytes from reticulocytes may be promoted by shear stress provided by the extracellular matrix. The extracellular matrix may contribute to maintaining the close proximity of pyrenocytes and macrophages. Pyrenocytes separated from reticulocytes expose PtdSer on their surface. Protein S in the serum functions as a bridge between pyrenocytes and macrophages by binding to the PtdSer on pyrenocytes and to MerTK on macrophages, and promotes the engulfment of pyrenocytes by the macrophages.
A model for definitive erythropoiesis on erythroblastic islands. Erythroblasts bind to a central macrophage through the interactions of integrin-α4β1 on erythroblasts and Vcam1 on macrophages. Erythroblasts then undergo enucleation, in which the separation of pyrenocytes from reticulocytes may be promoted by shear stress provided by the extracellular matrix. The extracellular matrix may contribute to maintaining the close proximity of pyrenocytes and macrophages. Pyrenocytes separated from reticulocytes expose PtdSer on their surface. Protein S in the serum functions as a bridge between pyrenocytes and macrophages by binding to the PtdSer on pyrenocytes and to MerTK on macrophages, and promotes the engulfment of pyrenocytes by the macrophages.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank K. Okamoto-Furuta and H. Kohda for help in electron microscopy, and M. Fujii for secretarial assistance. This work was supported in part by Grants-in-Aid from the Ministry of Education, Science, Sports, and Culture in Japan. S.T. is a research fellow of the Japan Society for the Promotion of Science.
Authorship
Contribution: S.T., K.S., and S.N. designed and analyzed the experiments, and wrote the manuscript; and S.T. performed the experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shigekazu Nagata, Department of Medical Chemistry, Graduate School of Medicine, Kyoto University, Yoshida, Sakyo-ku, Kyoto 606-8501, Japan; e-mail: snagata@mfour.med.kyoto-u.ac.jp.