Key Points
VTE recurrence risk in patients with cancer can be stratified by cancer type, stage, stage progression, and presence of leg paresis.
Patients with cancer at high VTE recurrence risk should be considered for secondary prophylaxis.
Abstract
Active cancer is the major predictor of venous thromboembolism (VTE) recurrence, but further stratification of recurrence risk is uncertain. In a population-based cohort study of all Olmsted County, Minnesota, residents with active cancer-related incident VTE during the 35-year period from 1966 to 2000 who survived 1 day or longer, we estimated VTE recurrence, bleeding on anticoagulant therapy, and survival and tested cancer and noncancer characteristics and secondary prophylaxis as predictors of VTE recurrence and bleeding, using Cox proportional hazards modeling. Of 477 patients, 139 developed recurrent VTE over the course of 1533 person-years of follow-up. The adjusted 10-year cumulative VTE recurrence rate was 28.6%. The adjusted 90-day cumulative incidence of major bleeding on anticoagulation was 1.9%. Survival was significantly worse for patients with cancer who had recurrent VTE (particularly pulmonary embolism) and with bleeding on anticoagulation. In a multivariable model, brain, lung, and ovarian cancer; myeloproliferative or myelodysplastic disorders; stage IV pancreatic cancer; other stage IV cancer; cancer stage progression; and leg paresis were associated with an increased hazard, and warfarin therapy was associated with a reduced hazard, of recurrent VTE. Recurrence rates were significantly higher for cancer patients with 1 or more vs no predictors of recurrence, suggesting these predictors may be useful for stratifying recurrence risk.
Introduction
Active cancer is associated with a two- to ninefold increased risk for recurrent venous thromboembolism (VTE).1-8 Moreover, the hazard of death is increased threefold among patients with cancer who have recurrent VTE, suggesting that prevention of VTE recurrence may be important for long-term survival.9,10 However, patients with cancer also have a high risk for anticoagulant-associated major bleeding,2,5,11-14 such that secondary prophylaxis for all patients with active cancer and incident VTE may be inappropriate. Independent predictors of VTE recurrence among patients with cancer are uncertain15 ; patient sex; brain cancer among women; lung, gastrointestinal, and genitourinary cancer; myeloproliferative disorders; tumor stage; adenocarcinoma; metastasis; and chemotherapy all have been suggested as predictors of VTE recurrence,1,5,7,9,15-18 but no studies have comprehensively tested all of these characteristics. To address this important gap in knowledge, we conducted a population-based historical cohort study of patients with active cancer and incident VTE to estimate VTE recurrence, estimate bleeding while receiving anticoagulation therapy, estimate survival after VTE recurrence and bleeding, and test baseline cancer and noncancer characteristics and secondary prophylaxis as potential predictors of VTE recurrence and bleeding.
Methods
Study setting, design, and population
Using the resources of the Rochester Epidemiology Project (see supplemental Appendix, available on the Blood Web site),19 we identified the inception cohort of all Olmsted County, Minnesota, residents with incident deep vein thrombosis (DVT), pulmonary embolism (PE), and/or chronic thromboembolic pulmonary hypertension (CTEPH) during the 35-year period from 1966 to 2000, as previously described.20,21 This study was confined to residents with active cancer-associated incident VTE during this period, defined as the presence of active cancer (see supplemental Appendix for definition of active cancer) within 92 days before or after the incident VTE event date. We followed each Olmsted County resident with incident VTE and active cancer, conditional on surviving 1 day, forward in time from the onset of incident VTE symptoms or signs to first DVT or PE recurrence (see supplemental Appendix for definition of VTE recurrence), using the patient’s complete (inpatient and outpatient) medical record while residing in the community.8,22 For deceased patients, all autopsy reports and death certificates were reviewed regardless of the location at death. The study was approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards. The study was conducted in accordance with the Declaration of Helsinki.
Measurements
Using explicit criteria, trained and experienced nurse abstractors reviewed all medical records in the community and collected data on date and type of incident VTE, baseline characteristics (age at incident VTE, sex, body mass index, hospitalization within the previous 3 months with or without surgery [categorized as general, neurological, orthopedic, cardiac, or gynecologic surgery], nursing home confinement, congestive heart failure, neurologic disease with leg paresis), dates of heparin and warfarin initiation and completion, inferior vena cava (IVC) filter placement, date and type of first VTE recurrence, and vital status at last clinical contact, as previously performed.1,8,22-24 Additional data were collected from the Mayo Clinic Tumor Registry (continuously operational since 1972) and from medical records on the date of diagnosis of active cancer, tumor site, tumor stage, tumor-node-metastasis classification and grade of cancer at the time of cancer diagnosis; presence of histology-proven biopsy; presence of venous invasion by cancer, lymph node involvement, and metastasis to liver and/or bone; tamoxifen therapy and chemotherapy (cytotoxic or immunosuppressive therapy for malignancy). For cancers without stage or grade, the study oncologists assigned stage and grade, where possible, on the basis of medical record review and knowledge of the cancer type or site (see supplemental Appendix for methods of assigning stage and grade). Cancer characteristics were recorded as present only if documented within the 3 months before or after the incident VTE event, and chemotherapy or radiotherapy characteristics were recorded as present if documented within the 3 months before the incident VTE event. All other cancer characteristics were recorded as present if documented any time before the incident VTE event. The study oncologists also restaged the cancer or cancers at the time of the incident VTE. All cancer stage assignments were adjudicated by the study oncologists and based on American Joint Committee on Cancer staging guidelines (sixth edition).25 Data also were collected on major and minor bleeding (see supplemental Appendix for definitions of major and minor bleeding) while receiving anticoagulation therapy.
Statistical analysis
In an initial analysis of all Olmsted County residents with active cancer-associated incident VTE during the 35-year period (1966-2000), we compared those who died on the day of the incident VTE event (including autopsy-discovered events) with those who survived 1 or more days, using the Wilcoxon rank-sum test for continuous variables and the χ-squared test, Fisher’s exact test, or a logistic model for categorical variables (see supplemental Appendix).
The endpoint for the primary analyses was the first recurrent VTE among those who survived 1 or more days. Data were censored at the first occurrence of the following: date last seen by a medical provider, death, or December 31, 2005. The cumulative incidence rates of first VTE recurrence and of survival after PE ± DVT vs DVT alone were estimated using the Kaplan-Meier estimator; separate analyses of the former were done with death as a competing risk (see supplemental Appendix).26 For the estimates of survival, data were censored at the first occurrence of either the date of last follow-up (vital status) or December 31, 2005.
Cancer type and stage may be confounded. For example, some cancers (pancreatic, ovarian) are nearly always diagnosed at a very late stage. To reduce the model dimensionality and potential confounding among covariates, we used the Akaike Information Criteria27 to choose a combined “stage by cancer type” variable (see supplemental Appendix). As part of this process, we separately examined those patients whose cancer stage was assigned by study oncologists.
Baseline characteristics were first examined in Cox proportional hazard (Cox PH) regression models unadjusted for any other variables. After adjusting for the combined cancer type and stage variable, we used a stepwise procedure to develop a multivariable model including all relevant baseline cancer and noncancer covariates. We then assessed the time-dependent variables of heparin and warfarin acute therapy and secondary prophylaxis. Finally, we examined all relevant interactions (see supplemental Appendix).
We also calculated the observed interval-specific event rates of recurrent VTE. The denominators were total person-years at risk in each interval of follow-up time among those with 1 or more days of follow-up. The numerators were the total numbers of recurrent VTE in the same follow-up time intervals.
The cumulative incidence rates of first major and of first major or minor bleeding event while receiving anticoagulation therapy were estimated using the Kaplan-Meier estimator. Data were censored at the first occurrence of the following: end of risk for anticoagulation-associated bleeding (defined as up to 7 days after anticoagulation was stopped); recurrent VTE; last follow-up by a medical provider; December 31, 2005; or death. We examined the relationship of the time-dependent variables of heparin and warfarin therapy and time to first bleeding event in a Cox PH model, both alone and adjusting for a collapsed version of the combined cancer/stage multilevel variable developed in the analysis of time to recurrent VTE (see supplemental Appendix).
To calculate the excess number of deaths resulting from recurrent VTE, we constructed separate univariate models in which recurrent VTE and major or minor bleed were treated as time-dependent variables in a Cox PH regression model of time to death. For each model, predicted cumulative survival probabilities were derived for each observed time point. Assuming complete follow-up, we multiplied the total number with incident VTE by the cumulative probability of death at each time point to get an estimated number of deaths assuming no recurrent VTE. We then subtracted this number from the actual number of deaths to calculate excess deaths resulting from recurrent VTE with anticoagulant treatment. To estimate the hypothetical number of recurrence-related deaths in the absence of warfarin treatment, we multiplied this number of excess deaths by the reciprocal of the Cox PH model-derived hazard ratio for predicting time to recurrent VTE, reasoning that removing the protective effect of warfarin would increase deaths due to recurrence by this proportion.
We assumed that all major or minor bleeds were preventable by not giving anticoagulant treatment. We estimated survival probabilities both with and without major or minor bleed, using the time-dependent bleeding variable, and compared the difference in the 2 survival probabilities at 90 days and multiplied this difference by the total number who had major or minor bleeds to calculate the estimated number of preventable bleeds.
Results
During the 35-year study period, 3385 Olmsted County residents were diagnosed with incident VTE (DVT alone, n = 1596; PE with or without DVT, n = 1784; CTEPH, n = 5), of whom 681 (20.1%) had active cancer. Of the 681 incident VTE cases with active cancer, 204 (30%) either had their VTE diagnosed solely at autopsy (n = 203) or died on the day of incident VTE diagnosis (n = 1) and were excluded from the analyses. Among the remaining 477 patients with active cancer, 56 (11.7%) had their cancer diagnosed within 3 months after the incident VTE event date and were included in the analyses; almost 80% of these cancers were diagnosed within 4 weeks after the incident VTE event, and 66% and 10.7% were stage IV/III and II, respectively, at diagnosis. For the 477 cases, the mean (standard deviation) and median (interquartile range [IQR]) patient ages at the incident VTE event date were 66.8 (14.7) and 68.9 (58.1-76.8) years, and 46.3% were women. Of these VTEs, 280 were DVT alone and 197 were PE ± DVT; DVT cases included arm (n = 22), mesenteric (n = 3), IVC (n = 3), ovarian (n = 2), and renal (n = 4) vein thrombosis (see supplemental Table 1 for a comparison of patients with active cancer who died on the day of the incident VTE vs survived ≥1 day). Of these 477 patients, 413 (87%) received at least 1 dose of heparin, and 366 (77%) received at least 1 dose of warfarin; 352 (74%) received both heparin and warfarin, and 45 (9%) received an IVC filter. The median duration of heparin anticoagulation was 5 days (IQR, 4-8; range, 1-427 days); 95% of patients received heparin for 20 days or less. The median duration of warfarin anticoagulation was 79 days (IQR, 18-166; range, 1-4864 days).
During 1533 person-years, 139 of 477 patients had VTE recurrence (64 PE ± DVT and 75 DVT alone), and 303 (89.6%) of the 338 who did not recur died. The recurrent DVT cases included arm (n = 8), IVC (n = 2), mesenteric (n = 1), and renal (n = 1) vein thrombosis. The median of the recurrence times was 92 days (IQR, 26-751; range, 1 day to 33 years). The cumulative VTE recurrence rates at 1 week, 1 month, 3 months, 6 months, 1 year, 5 years, and 10 years were 1.6%, 10.3%, 18.0%, 21.4%, 26.7%, 45.0%, and 52.2%, respectively (Table 1, which also shows the corresponding interval-specific recurrence rates); recurrence rates were significantly higher for patients with active cancer compared to patients without active cancer (Figure 1). The cumulative mortality at 3 months, 2 years, and 10 years were 33.8%, 66.4%, and 85.2%, respectively (Table 1). As a consequence, the 10-year cumulative VTE recurrence rate, allowing for the competing risk for death, was 28.6%. Among those with recurrent PE ± DVT, the cumulative mortality at 7, 30, 60, and 90 days was 32.8%, 42.2%, 51.6%, and 67.2% (supplemental Table 2). Among those with recurrent DVT alone, the corresponding cumulative mortality was 2.7%, 2.7%, 17.3%, and 30.7%. Mortality was significantly worse after recurrent PE ± DVT compared with recurrent DVT alone (P ≤ .001, log-rank test; supplemental Figure 1).
Cumulative incidence of first VTE recurrence among Olmsted County, Minnesota, residents with incident DVT or PE, 1966-2000, and followed-up through December 31, 2005, by active cancer status. The “no active cancer group” includes incident VTE cases with prior cancer that was inactive (see supplemental Appendix) at the time of the incident VTE event.
Cumulative incidence of first VTE recurrence among Olmsted County, Minnesota, residents with incident DVT or PE, 1966-2000, and followed-up through December 31, 2005, by active cancer status. The “no active cancer group” includes incident VTE cases with prior cancer that was inactive (see supplemental Appendix) at the time of the incident VTE event.
Initial modeling of cancer site and stage as potential predictors of VTE recurrence identified a model that included stage IV pancreatic cancer (only 4 [14.3%] of 28 pancreatic cancer patients were not stage IV); other stage IV cancer; brain, ovarian, lung, and noncolorectal gastrointestinal cancer (stomach, liver, other digestive cancer); myeloproliferative or myelodysplastic disorders; and a combined group of stage III cancer, acute lymphocytic leukemia (ALL), or acute myelocytic leukemia (AML) as the most parsimonious model of significant site/stage predictors of recurrence (Table 2; supplemental Appendix; supplemental Tables 3-5). In univariate analyses of other cancer characteristics as potential predictors of VTE recurrence, both liver and other metastases vs no metastases and multiple active cancers were significant predictors of recurrence, whereas cancer stage progression, cancer histology and grade, vein invasion by cancer, chemotherapy and radiation therapy, and tamoxifen were not univariately associated with VTE recurrence (Table 2; supplemental Tables 6-7). In univariate analyses of noncancer baseline and time-dependent characteristics, unadjusted for the combined malignancy site/stage variable, neurological disease with leg paresis and neurosurgery within the 3 months before the incident VTE event were significant predictors of VTE recurrence (Table 2). The hazard of recurrence with warfarin therapy was significantly reduced. Including all treatment information, 53 (38.1%) patients had a treatment failure at the time of their recurrent event.
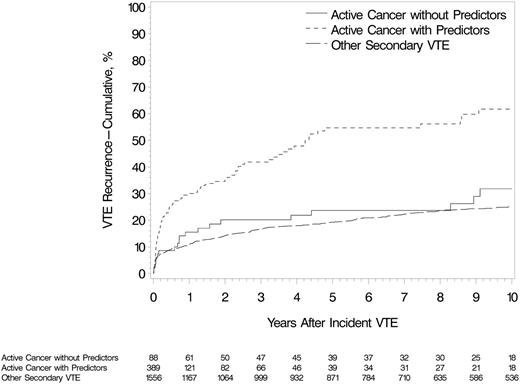
In the multivariate model, stage IV pancreatic cancer, brain cancer, myeloproliferative or myelodysplastic disorders, ovarian cancer, stage IV cancer (nonpancreatic), lung cancer, neurological disease with leg paresis, and cancer stage progression were independent, significant, and clinically important predictors of VTE recurrence (Table 3; supplemental Table 8). Multiple active cancers, noncolorectal gastrointestinal cancer, and the combined group of stage III cancer, ALL, or AML were marginally predictive of recurrence, with hazard ratios ranging from 1.9 to 1.5. Secondary prophylaxis with warfarin was significantly protective against VTE recurrence, with a 60% reduced hazard. In a multivariate model that included all independent predictors of VTE recurrence (Table 3), tamoxifen was not a predictor of VTE recurrence (hazard ratio [HR], 1.57; 95% confidence interval [CI], 0.71-3.45; P = .26). Among those with incident cancer-associated VTE, patients with 1 or more predictors of recurrence (from Table 3) had a significantly increased risk for recurrent VTE compared with patients with none of these predictors (HR, 3.02; 95% CI, 2.43-3.76; P < .001; Figure 2). The latter group had a nonsignificant higher risk for recurrent VTE compared with those with incident noncancer secondary VTE (HR, 1.4; 95% CI, 0.9-2.1; P = .16; Figure 2). Of the 477 patients with active cancer, 78% had an objectively documented incident VTE event, and 71.2% of recurrent events were objectively documented. The results of analyses limited to cases with objectively documented recurrent events were not substantially changed (supplemental Table 9). Adjusting for age, sex, and the above cancer characteristics associated with recurrence, recurrent VTE increased the hazard of death almost threefold (HR, 2.7; 95% CI, 2.1-3.4).
Cumulative incidence of first VTE recurrence among Olmsted County, Minnesota, residents with incident DVT or PE, 1966-2000, associated with active cancer and 1 or more predictor of venous thromboembolism recurrence (from Table 3), active cancer and no predictor of venous thromboembolism recurrence, and noncancer secondary VTE (see supplemental Appendix).
Cumulative incidence of first VTE recurrence among Olmsted County, Minnesota, residents with incident DVT or PE, 1966-2000, associated with active cancer and 1 or more predictor of venous thromboembolism recurrence (from Table 3), active cancer and no predictor of venous thromboembolism recurrence, and noncancer secondary VTE (see supplemental Appendix).
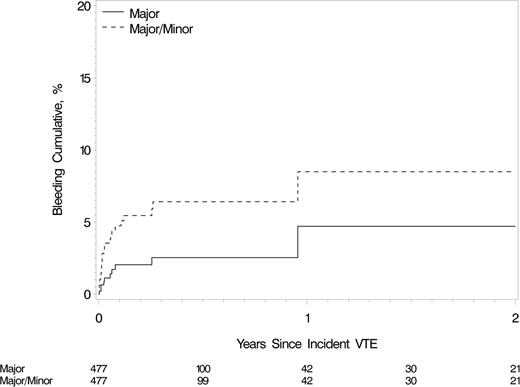
There were 11 first major bleeding events while receiving anticoagulation therapy; 8 occurred within the first 30 days of treatment, 3 of which were fatal (supplemental Table 10). The cumulative incidence of major bleeding on anticoagulation at 7, 14, 30, 90, and 183 days and 1 year were 0.6%, 1.1%, 2.0%, 2.0%, 2.5%, and 4.7%, respectively (Figure 3). Allowing for the competing risk for death, the 1-year cumulative incidence of major bleeding was 4.0%. There were 26 major or minor bleeding events on anticoagulation; 50% occurred within the first 7 days of treatment. The cumulative incidence of major or minor bleeding on anticoagulation at 7, 14, 30, 90, and 183 days and 1 year were 2.8%, 3.5%, 4.7%, 5.4%, 6.4%, and 8.5%, respectively (Figure 3). Allowing for the competing risk for death, the 1-year cumulative incidence of major or minor bleeding was 7.5%. Two patients developed VTE recurrence at 3 and 10 days, respectively, after anticoagulation was stopped for major bleeding; both patients died at 1 day and 2 weeks, respectively, after VTE recurrence. Major or minor bleeding increased the hazard of death more than twofold (HR, 2.3; 95% CI, 1.5-3.5). In analyses of potential predictors of major or minor bleeding while receiving anticoagulation therapy, the hazard was significantly increased for heparin (HR, 3.6; P < .01), but not for warfarin therapy.
Cumulative incidence of first major bleeding event and first major or minor bleeding event while receiving anticoagulation therapy among Olmsted County, Minnesota, residents with incident active cancer-associated VTE, 1966-2000, and followed-up through December 31, 2005.
Cumulative incidence of first major bleeding event and first major or minor bleeding event while receiving anticoagulation therapy among Olmsted County, Minnesota, residents with incident active cancer-associated VTE, 1966-2000, and followed-up through December 31, 2005.
Discussion
We found a 52% 10-year cumulative incidence of VTE recurrence among patients with active cancer. From the perspective of the patient (who expects to survive), these rates are most important for counseling regarding secondary prophylaxis. However, because of very poor survival, the cumulative recurrence rate was only about half as much (29%) after allowing for the competing risk for death. The latter figure may be more relevant from a health care policy perspective. Of particular note, we found that survival was significantly lower after recurrent PE ± DVT compared with DVT alone. Given that incident PE is an independent predictor of reduced survival among cancer patients,13,28-32 preventing PE recurrence among patients with active cancer may improve survival.9,10,33
Patients with active cancer also are reportedly at high risk for anticoagulant-associated bleeding,2,5,9,12-14 such that after the 3-month acute treatment phase is completed,34,35 one would prefer to target secondary prophylaxis to those cancer patients at high risk for recurrence and avoid the risk for anticoagulant-associated bleeding in low-recurrence-risk patients. We found brain, lung, and ovarian cancer; myeloproliferative or myelodysplastic disorders; stage IV pancreatic cancer; other stage IV cancer; cancer stage progression; and leg paresis to be independent predictors of an increased hazard for VTE recurrence among patients with active cancer, whereas secondary prophylaxis with warfarin independently reduced the hazard of recurrence. Our findings are supported by previous studies that reported an increased risk for recurrence among patients with brain cancer,5,9 lung cancer,5,9,15,18 and myeloproliferative or myelodysplastic disorders.16 Although the risk for incident VTE is increased for patients with pancreatic36,37 and ovarian cancer,38 we are the first to identify these 2 cancer sites as independent predictors of VTE recurrence. Most (86%) of our incident VTE cases with pancreatic cancer had stage IV cancer, such that we could not separate the effects of advanced cancer stage and pancreatic cancer on the hazard of VTE recurrence. However, stage IV pancreatic cancer had nearly a fourfold increased hazard of recurrence (HR, 3.8; 95% CI, 1.68-8.58; P = .001) compared with all other stage IV cancers, suggesting that pancreatic cancer is a high-risk cancer for VTE recurrence.
Among other active cancers, stage IV cancer was an independent predictor of VTE recurrence. This finding is supported by a post hoc analysis of a clinical trial and a systematic review that found an increased recurrence risk among cancer patients with metastatic cancer compared with localized disease.15,39 A systematic review that included 2 evaluable studies found a 1.4-fold increased risk for recurrence for metastasis compared with no metastasis, but available data were insufficient to control for cancer site or stage.15 Although most of our patients with stage IV cancer had metastatic disease, we also categorized acute leukemias and brain cancer as “stage IV” cancer for the purposes of analyses (see supplemental Appendix), which likely accounts for our identification of stage IV cancer rather than “metastases” or “metastases location” as independent predictors of recurrence. To our knowledge, we are the first to identify cancer stage progression and neurological disease with leg paresis as independent predictors of VTE recurrence among patients with active cancer; leg paresis is a known risk factor for incident VTE.1 Although recent neurosurgery before the incident VTE event was a univariate predictor of recurrence, 40% of patients with brain cancer had recent neurosurgery before their incident VTE such that, after adjusting for brain cancer, neurosurgery was not a predictor of recurrence.
Secondary prophylaxis with warfarin significantly reduced the hazard of VTE recurrence by about 60%. The low median duration of warfarin anticoagulation (79 days) was a result of poor patient survival. Because prothrombin time/international normalized ratio data were not available on all patients receiving warfarin, we could not adjust for time in therapeutic range. However, adjustment for time in therapeutic range could only affect the magnitude of the hazard of VTE recurrence associated with warfarin anticoagulation. The seminal study demonstrating the superior efficacy of low-molecular-weight heparin (LMWH) over vitamin K antagonists in preventing VTE recurrence among patients with active cancer was published in 2003.40 Thus, essentially none of our VTE cases received acute treatment or secondary prophylaxis with LMWH. Although LMWH secondary prophylaxis is recommended over warfarin,41 many patients are unable to afford or tolerate parenteral LMWH. Our findings show that warfarin secondary prophylaxis is an effective (albeit, secondary) alternative to LMWH.
Cancer grade and histology, chemotherapy and type of chemotherapy, central venous catheter, radiation therapy, and venous invasion were not independent predictors of VTE recurrence. A population-based prospective cohort study of 147 patients with active cancer with VTE found a nonsignificant higher rate of recurrence among patients with adenocarcinoma (22%) compared with other cancer histologies (17%).7 We previously reported a higher hazard for recurrence among patients with cancer-associated incident VTE who were receiving cytotoxic or immunosuppressive chemotherapy (HR, 4.24) compared with a similar patient who was not receiving chemotherapy (HR, 2.21), but we did not control for cancer site or stage in that analysis.1
Our finding of an age- and sex-adjusted 1-year major or minor bleeding rate of 8.5% is similar to previously reported rates of 12.4% to 13.3%;2,5 allowing for the competing risk for death, this incidence was about 12% lower (7.5%). Our adjusted 90-day major bleeding incidence (1.9%) was similar to the 1.6% incidence considered as “low risk” by recent ACCP guidelines.41 We found the hazard of major or minor bleeding was significantly increased for heparin anticoagulation early in the acute treatment phase. Given that 50% of all major or minor bleeding events occurred within the first 7 days of treatment, heparin therapy may unmask an underlying predistribution to bleeding. In additional modeling (data not shown), brain cancer, noncolon gastrointestinal cancer, and multiple active cancers had hazards of bleeding more than threefold higher than the comparison group but were not statistically significant (P > .20).
There is always a tension between preventing VTE recurrence and causing bleeding with anticoagulation therapy. We estimated that in the absence of anticoagulation therapy, 35 additional deaths from recurrent VTE and 6 fewer deaths from bleeding would have occurred (see supplemental Appendix). Given that LMWH is more efficacious than warfarin, with no significant difference in bleeding,38 the number of deaths averted by LMWH therapy might be greater. These analyses may help to inform decisions regarding anticoagulation therapy.
Our results are likely to be valid. Because of our large cohort sample size and long follow-up duration, it is unlikely that we failed to identify important predictors of recurrence because of inadequate power. We also avoided the potential distortions associated with referral bias and an incomplete spectrum of disease by performing a population-based study that included all residents from a well-defined geographic area with active cancer-associated incident VTE. We accurately separated incident from recurrent VTE events and used an unambiguous definition of VTE recurrence. For deceased patients, all death certificates and autopsy reports were reviewed regardless of the location at death. Although cancer chemotherapy has changed during the 35-year period of our study, cancer chemotherapies, both overall and by chemotherapy category (ie, alkylators and antimetabolites, anthracyclines, plant alkaloids and platinums, and hormone, immunomodulators, and others; supplemental Table 7) were not independent predictors of VTE recurrence. We estimated VTE recurrence over 2 time frames, which represented milestones in the introduction of new chemotherapy agents (1966-1985 [introduction of anthracycline] and 1986-2000 [introduction of hematopoietic growth factors and targeted therapies, such as rituximab, trastuzumab, imatinib]) and found no significant difference in recurrence between these 2 timeframes (P = .39; supplemental Figure 2). Finally, VTE event year was not a univariate predictor of VTE recurrence (HR, 0.99; 95% CI, 0.97-1.01; P = .16). However, although our results likely are relevant to current care, there may be additional patients with cancer at high risk for VTE recurrence (eg, patients with multiple myeloma receiving immunomodulatory therapy), and VTE recurrence rates may differ with LMWH therapy and newer cancer therapies.
It is also important to address potential limitations of our study. Because the racial and ethnic demography of Olmsted County is predominantly white of non-Hispanic ancestry and LMWH and novel oral anticoagulants were not yet available, our findings may not be generalizable to populations of other races or ethnicities or to other treatments.
In conclusion, tumor site, stage, and stage progression are independent predictors of VTE recurrence. In particular, patients with brain, lung, stage IV pancreatic, or ovarian cancer; myeloproliferative or myelodysplastic disorders; stage IV cancer; cancer stage progression; or leg paresis have the highest risk for recurrence and should be considered for secondary prophylaxis. Although secondary prophylaxis with LMWH is preferred, warfarin prophylaxis does significantly reduce the hazard of VTE recurrence by about 60%.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We gratefully acknowledge Catherine L. Brandel, Diadra H. Else, Jane A. Emerson, and Cynthia L. Nosek for excellent data collection and Cynthia E. Regnier as research project manager. Research reported in this publication was supported by a grant from the National Institutes of Health, National Heart Lung and Blood Institute (R01HL66216 to J.A.H.) and was made possible by the Rochester Epidemiology Project (National Institutes of Health, National Institute on Aging (R01AG034676 ). Research support also was provided by Mayo Foundation.
Authorship
Contribution: C.E.C. collected, analyzed, and interpreted the data and wrote the manuscript; A.A.A. and R.S.M. collected, analyzed, and interpreted the data and contributed to the manuscript; T.M.P. designed and performed the research, collected data, performed the statistical analyses, and contributed to the manuscript; K.R.B. directed the statistical analyses and contributed to the manuscript; L.J.M. contributed to the research design, analyzed and interpreted the data, and contributed to the manuscript; and J.A.H. designed and performed the research, collected data, analyzed and interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: J.A.H. has participated in advisory boards for Daiichi Sankyo and Janssen Pharmaceuticals, for which he has received honoraria. The remaining authors declare no competing financial interests.
Correspondence: John A. Heit, Stabile 6-Hematology Research, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: heit.john@mayo.edu.