To the editor:
We read with great interest the article by Saze et al,1 which was published recently in Blood as a plenary paper. The authors illustrated that human peripheral blood B cells express ectonucleotidases CD39 and CD73. Thus, B cells are capable of hydrolyzing adenosine triphosphate to adenosine 5′-monophosphate (5′-AMP) via CD39 and subsequently to adenosine (ADO) by means of CD73 enzymatic activity.
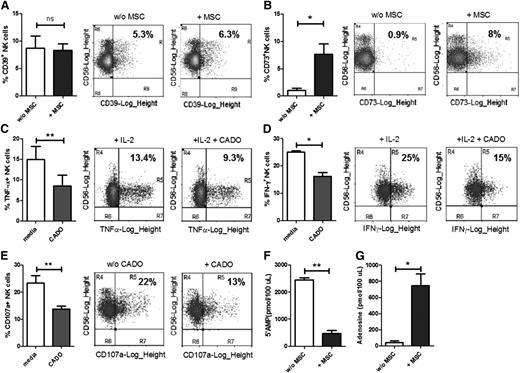
In our laboratory, we study the interaction between human natural killer (NK) cells and mesenchymal stem cells (MSCs). Due to their immunosuppressive properties and inherent immunopriviledged nature, MSCs can be used as biological tolerance inducers in inflammatory and autoimmune diseases.2,3 In this work, we cocultured fluorescence-activated cell sorter (FACS)-sorted allogeneic NK cells with human umbilical cord (UC)-derived MSCs obtained from consenting donors. We found that freshly isolated NK cells express CD39, and the level of CD39 expression remains unaltered on coculture with MSCs (Figure 1A). The percentage of CD73-expressing NK cells in peripheral blood is very low (∼1%). However, on coculture with MSCs, the percentage of CD73+ NK cells increases significantly (Figure 1B). Thus, a subpopulation of NK cells acquires the ability to convert AMP into ADO on exposure to MSCs.
CD39 and CD73 expression on NK cells and the effect of ADO analog on NK cell functions. Blood samples were obtained from healthy volunteers, and peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll density gradient centrifugation. CD56+CD3− NK cells were sorted out from PBMCs using FACS and cultured overnight in the presence/absence of MSCs. (A) CD39 and (B) CD73 expression was analyzed on the NK cells using flow cytometry. (C-D) NK cells were stimulated with 1000 IU/mL interleukin-2 (IL-2) in the presence/absence of 15 µM CADO and incubated overnight. Brefeldin A was added to the cultures after 1 hour of incubation. The cells were then washed, fixed, and stained intracellularly for (C) tumor necrosis factor (TNF)-α or (D) interferon (IFN)-γ. (E) NK cells were cultured with/without 15 µM CADO overnight, washed, and further incubated with K562 cells for 4 hours. CD107a surface expression was analyzed on the NK cells as a readout for NK cell degranulative capacity. (F-G) NK cells cultured with or without MSCs were cultivated in PBS with 5′AMP as a substrate for 30 minutes at 37°C. Cell-free supernatants were collected and analyzed using chromatography with tandem mass spectrometry for the levels of (F) residual 5′-AMP (as a readout of substrate utilization) or (G) ADO (as a read out of product accumulation). Paired 2-tailed Student t tests were performed using GRAPHPAD PRISM V5.00 software. Levels of significance are shown as P values (*P < .05, **P < .01). Bar graphs represent mean ± standard error of mean.
CD39 and CD73 expression on NK cells and the effect of ADO analog on NK cell functions. Blood samples were obtained from healthy volunteers, and peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll density gradient centrifugation. CD56+CD3− NK cells were sorted out from PBMCs using FACS and cultured overnight in the presence/absence of MSCs. (A) CD39 and (B) CD73 expression was analyzed on the NK cells using flow cytometry. (C-D) NK cells were stimulated with 1000 IU/mL interleukin-2 (IL-2) in the presence/absence of 15 µM CADO and incubated overnight. Brefeldin A was added to the cultures after 1 hour of incubation. The cells were then washed, fixed, and stained intracellularly for (C) tumor necrosis factor (TNF)-α or (D) interferon (IFN)-γ. (E) NK cells were cultured with/without 15 µM CADO overnight, washed, and further incubated with K562 cells for 4 hours. CD107a surface expression was analyzed on the NK cells as a readout for NK cell degranulative capacity. (F-G) NK cells cultured with or without MSCs were cultivated in PBS with 5′AMP as a substrate for 30 minutes at 37°C. Cell-free supernatants were collected and analyzed using chromatography with tandem mass spectrometry for the levels of (F) residual 5′-AMP (as a readout of substrate utilization) or (G) ADO (as a read out of product accumulation). Paired 2-tailed Student t tests were performed using GRAPHPAD PRISM V5.00 software. Levels of significance are shown as P values (*P < .05, **P < .01). Bar graphs represent mean ± standard error of mean.
ADO has been shown to suppress inflammation by regulating various immune cells.4,5 Saze et al1 also showed that 2-chloroadenosine (CADO), a stable ADO analog, could suppress B-cell functions like cytokine production, suggesting an autocrine regulatory role for ADO in B-cell physiology. To determine whether CADO also affects NK cell function, we cultured NK cells overnight with 1000 IU/mL of IL-2 in the presence/absence of 15 μM CADO. We observed that CADO significantly suppresses IL-2 induced tumor necrosis factor-α and interferon-γ production by NK cells (Figure 1C-D). We also studied the effect of overnight CADO treatment on unstimulated NK cells. When these CADO pretreated NK cells were exposed to K562 leukemic cells, the former exhibited significantly reduced degranulation capacity compared with NK cells cultured without CADO (Figure 1E).
Finally, using mass spectroscopy, we could demonstrate that up-regulation of ectonucleotidase CD73 on NK cells is also accompanied by increased enzyme activity (Protocol as described in supplemental Material 1). First, we could document increased utilization of the CD73 substrate 5′-AMP by NK cells that were precultured with MSCs (Figure 1F). Second, we could directly establish that NK cells precultivated with MSCs were able to produce significantly higher amounts of ADO compared with NK cells cultured alone (Figure 1G).
MSCs have the ability to home to sites of inflammation in vivo.6,7 NK cells interacting with these MSCs are likely to acquire CD73 expression. These CD73+ NK cells have the potential to regulate NK cell activation in an autocrine or paracrine manner. This might have widespread implications for immunomodulation in the inflammatory microenvironment.
The online version of this article contains a data supplement.
Authorship
Acknowledgments: This work was supported by the Deutsche Forschungsgemeinschaft (DFG; German Research Foundation), Sonderforschungsbereich, grant 738/A5, REBIRTH (Regenative Biology to Reconstructive Therapy) by the Cluster of Excellence from the DFG, and Niedersächsische Krebsgesellschaft.
Contribution: D.C., D.M.T., H.B., R.H., and R.J. performed experiments; D.C., H.B., and R.J. analyzed the results and made the figures; D.C., R.E.S., and R.J. designed the research; and D.C. wrote the letter.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Roland Jacobs, Department of Clinical Immunology and Rheumatology, Hannover Medical School, Carl-Neuberg-Strasse 1, 30625 Hannover, Germany; e-mail: jacobs.roland@mh-hannover.de.