Key Points
Eosinophils recruited to the airways in response to A fumigatus sensitization and challenge degranulate in response to virus infection.
Activated eosinophils are antiviral and promote survival from an otherwise lethal respiratory virus infection.
Abstract
Eosinophils are recruited to the airways as a prominent feature of the asthmatic inflammatory response where they are broadly perceived as promoting pathophysiology. Respiratory virus infections exacerbate established asthma; however, the role of eosinophils and the nature of their interactions with respiratory viruses remain uncertain. To explore these questions, we established acute infection with the rodent pneumovirus, pneumonia virus of mice (PVM), in 3 distinct mouse models of Th2 cytokine–driven asthmatic inflammation. We found that eosinophils recruited to the airways of otherwise naïve mice in response to Aspergillus fumigatus, but not ovalbumin sensitization and challenge, are activated by and degranulate specifically in response to PVM infection. Furthermore, we demonstrate that activated eosinophils from both Aspergillus antigen and cytokine-driven asthma models are profoundly antiviral and promote survival in response to an otherwise lethal PVM infection. Thus, although activated eosinophils within a Th2-polarized inflammatory response may have pathophysiologic features, they are also efficient and effective mediators of antiviral host defense.
Introduction
Asthma is a chronic inflammatory disease of the respiratory tract that is characterized by reversible airways hyper-responsiveness. Although the disease is heterogeneous in nature,1,2 eosinophilic leukocytes are typically identified as components of airway infiltrates in childhood asthma3 and are particularly numerous in the recently defined eosinophilic asthma phenotype.4,5 Mouse models of allergic airways inflammation suggest that eosinophils promote pathology, including mucus accumulation, airway hyper-responsiveness, and tissue remodeling.6,7 Respiratory viruses play a major part in promoting asthma exacerbations; meanwhile, several studies have documented a role for eosinophils in limiting virus infectivity and promoting virion clearance.8,9 Among these, our group has shown that the eosinophil-derived neurotoxin/ribonuclease 2 (EDN/RNase 2) and eosinophil cationic protein, major eosinophil secretory mediators detected in lung tissue of respiratory syncytial virus (RSV)-infected infants,10 can reduce the infectivity of this virus for target epithelial cells.11,12 Likewise, Adamko et al13 found that eosinophils recruited in response to ovalbumin (ova) sensitization promoted virus clearance in a guinea pig model, and Phipps et al14 reported more rapid clearance of RSV virions in hypereosinophilic interleukin (IL)-5 transgenic mice. However, whether eosinophils can provide significant protection against a replicating virus that elicits substantial morbidity in vivo remains uncertain.
To explore this possibility, we evaluated antiviral responses in 3 distinct models of Th2-driven eosinophilic airway inflammation followed by infection with pneumonia virus of mice (PVM; family Paramyxoviridae), a natural mouse pathogen that is closely related to RSV that elicits pathology similar to the more severe forms of RSV disease in human infants.15-17 Unlike RSV when used in rodent challenge models, PVM undergoes robust replication in mouse bronchial epithelial cells in vivo, elicits severe morbidity and mortality in inbred strains of mice, and fulfills Koch’s postulates for an infectious agent in a rodent host.18 We examined virus recovery and survival in the unique eotaxin-2/IL-5 double transgenic (B6-E2IL5tg) mouse model of bronchopulmonary inflammation, which exhibits remodeling and airways hyper-responsiveness accompanied by profound eosinophil activation and degranulation,19 analogous to that observed in chronic human asthma.20,21 We also examined virus recovery in PVM-infected, ova and Aspergillus fumigatus (Af)-sensitized and -challenged mouse models that likewise feature eosinophil recruitment to the airways.
Methods
Mouse strains
C57BL/6 (B6) mice were purchased from Division of Cancer Therapeutics, National Cancer Institute (Frederick, MD). B6-E2IL5tg19 mice were maintained via crosses between B6-E2IL5tg male and B6-E2tg female mice. Eosinophil-deficient B6-E2IL5tg-ΔdblGATA mice were generated by backcrossing B6-E2IL5tg mice into the eosinophil-deficient ΔdblGATA22 strain also on the C57BL/6 background. All mouse studies were approved by the National Institute of Allergy and Infectious Diseases and carried out in accordance with Animal Care and Use Committee Guidelines.
Virus
Tissue culture infectious dose (TCID)50 assays provided quantitative evaluation of infectious mouse-passaged PVM J3666 stocks.18 Infections were established in isoflurane-anesthetized mice via intranasal inoculation with 102 TCID50 units in 50 μL diluent.
Ova or Af sensitization and challenge
B6 mice were sensitized on days 28 and 14 via intraperitoneal injection with 20 µg Af antigens (Hollister Stier) or days 33 and 19 with 50 µg ova (Sigma-Aldrich) in ImjectAlum (Pierce; 100 µL/mouse) and challenged on days 3, 2, and 1 via intranasal inoculation with 25 µg Af or 50 µg ova in phosphate-buffered saline (PBS). Control mice were sensitized with Af or ova, respectively, in ImjectAlum and challenged with intranasal PBS only. Sensitized and challenged mice were inoculated with PVM J3666 or diluent as above on day 0 and evaluated on day 4 unless otherwise indicated.
Bronchoalveolar lavage and cell counts
Cytospins were prepared from bronchoalveolar lavage (BAL) fluid (1.5 mL in PBS with 0.1% bovine serum albumin), fixed, and stained with Diff-Quik.
Histology
Lungs of euthanized mice were inflated trans-tracheally using 250 μL 10% phosphate-buffered formalin. The lungs and heart were removed and fixed overnight in 10% phosphate-buffered formalin at 4°C. Samples were paraffin-embedded, sectioned, and stained with hematoxylin and eosin (Histoserv, Germantown, MD). Images were evaluated with a Leica DMI 4000B microscope and photographed with a Retiga model 2000R imaging system at magnifications indicated in the figure legends.
Electron microscopy
Cells were isolated from BAL fluid from Af-sensitized and -challenged, PVM-infected mice as described above. Cells were washed once in PBS and fixed overnight at 4°C with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.2. Samples were postfixed for 30 minutes with 0.5% osmium tetroxide/0.8% potassium ferricyanide, 1 hour with 1% tannic acid, and overnight with 1% uranyl acetate at 4°C. Samples were dehydrated with a graded ethanol series and embedded in Spurr’s resin. Thin sections were cut with an RMC MT-7000 ultramicrotome (Ventana) and stained with 1% uranyl acetate and Reynold lead citrate before viewing at 120 kV on a Tecnai BT transmission electron microscope (FEI). Digital images were acquired with a Hammamatsu XR-100 bottom mount CCD system (Advanced Microscopy Techniques) and processed with the use of Adobe Photoshop, version CS2 (Adobe Systems).
Detection of mouse eosinophil peroxidase
The enzyme-linked immunosorbent assay to detect mouse eosinophil peroxidase (mEPX) was performed on BAL fluid as previously described.23
Detection of mouse eosinophil-associated ribonucleases in BAL fluid
BAL fluid was subjected to gel electrophoresis and transfer by standard methods. After transfer, the blocked membrane was probed with a 1:200 dilution of rabbit-anti-mouse eosinophil-associated ribonucleases (mEars) polyclonal antibody24 followed by a 1:1000 dilution of alkaline phosphatase–conjugated goat anti-rabbit immunoglobulin and developing reagents. Ribonuclease activity was evaluated by spectrophotometric assay,25 which permits detection of acid-soluble ribonucleotides (OD260) generated at room temperature in 10 minutes from acid-insoluble transfer RNA substrate (Sigma-Aldrich; 20 mg/mL).
Isolation of total lung RNA for virus recovery
Virus recovery was determined from complementary DNA generated from total RNA from mouse lung tissue via a dual standard curve quantitative reverse transcriptase-polymerase chain reaction method targeting the PVM SH gene and transcript and mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as previously described.26
Isolation of eosinophils from airways and adoptive transfer by intratracheal inoculation
Total cells (∼100% eosinophils) harvested from BAL from B6-E2IL5tg mice (3.5-5.2 × 106 eosinophils/mouse) were collected, washed in PBS, and resuspended in 100 µL PBS with 0.1% bovine serum albumin. Cells or diluent were inoculated trans-tracheally into B6-E2IL5tg-ΔdblGATA mice anesthetized with ketamine-xylazine. Immediately thereafter, both groups received an intranasal inoculation of 102 TCID units PVM J3666 in 50 µL diluent and were evaluated 4 days later.
Statistical analysis
Data were analyzed via appropriate algorithms within GraphPad PRISM with one or more experimental repetitions.
Results
Constitutively degranulating airway eosinophils in the B6-E2IL5tg asthmatic inflammation model are antiviral and promote survival in response to an otherwise lethal respiratory virus infection
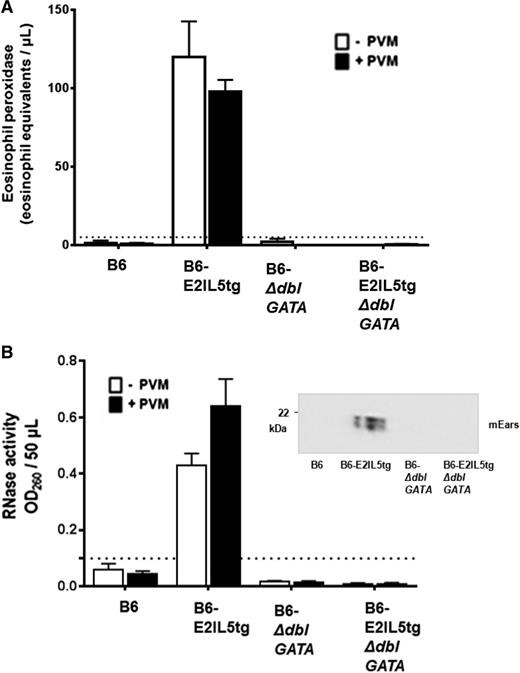
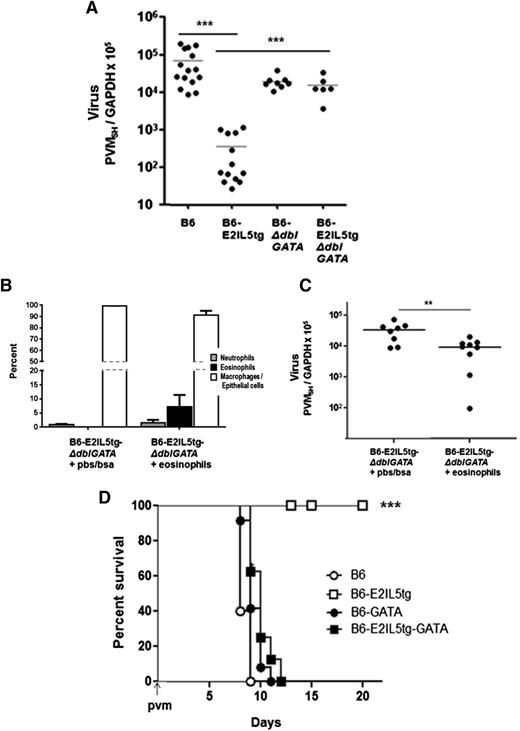
To examine eosinophil-mediated antiviral host defense, PVM infection was established in wild-type C57BL/6 (B6) mice, in B6-E2IL5tg19 mice, in eosinophil-deficient B6-ΔdblGATA mice,22 and in mice in which both eotaxin-2 and IL-5 transgenes were introduced into the B6-ΔdblGATA to create cytokine-enriched, eosinophil-deficient B6-E2IL5tg-ΔdblGATA controls. Eosinophils were prominent in the airways of the B6-E2IL5tg mice (106 cells/mouse, ∼100% eosinophils) but were few or absent in the airways of the other mouse strains (Figure 1A-B). Differential recruitment of eosinophils can also be observed in the histopathologic images of virus-infected mouse lungs. Eosinophils are dominant in the parenchyma and airspaces in the B6-E2IL5tg mice (Figure 1C-D). No eosinophils are detected in the B6-E2IL5tg-ΔdblGATA controls, which display foci of mild alveolar congestion typical of PVM infection in B6 mice at this time point (Figure 1E-F).
PVM infection in the cytokine-driven B6-E2IL5tg mouse model of airway inflammation. (A) Total eosinophils in BAL fluid from PVM-infected C57BL/6 (B6) wild-type, B6-eotaxin-2/IL-5 double transgenic (B6-E2IL5tg), eosinophil-deficient B6-ΔdblGATA, and cytokine-enriched eosinophil-deficient B6-E2IL5tg-ΔdblGATA mice; t = 4 days after PVM inoculation; n = 4 to 6 mice per time point; ***P < .0001 (1-way analysis of variance [ANOVA]). (B) Cell differentials of mice described in A. (C-D) Microscopic histology of lung tissue from PVM-infected B6-E2IL5tg mice; original magnifications ×10 and ×40, respectively. (E-F) Microscopic histology of lung tissue from PVM-infected B6-E2IL5tg-ΔdblGATA mice; original magnifications ×10 and ×40, respectively; area within box in (E) magnified as shown in (F).
PVM infection in the cytokine-driven B6-E2IL5tg mouse model of airway inflammation. (A) Total eosinophils in BAL fluid from PVM-infected C57BL/6 (B6) wild-type, B6-eotaxin-2/IL-5 double transgenic (B6-E2IL5tg), eosinophil-deficient B6-ΔdblGATA, and cytokine-enriched eosinophil-deficient B6-E2IL5tg-ΔdblGATA mice; t = 4 days after PVM inoculation; n = 4 to 6 mice per time point; ***P < .0001 (1-way analysis of variance [ANOVA]). (B) Cell differentials of mice described in A. (C-D) Microscopic histology of lung tissue from PVM-infected B6-E2IL5tg mice; original magnifications ×10 and ×40, respectively. (E-F) Microscopic histology of lung tissue from PVM-infected B6-E2IL5tg-ΔdblGATA mice; original magnifications ×10 and ×40, respectively; area within box in (E) magnified as shown in (F).
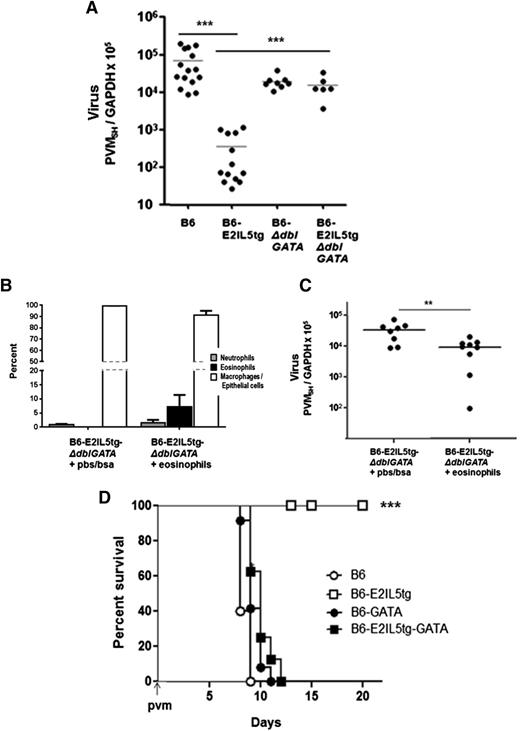
We observed a substantial, 1.7 to 2.3 1og reduction in virus recovery from the eosinophil-enriched B6-E2IL5tg mice compared with any of the aforementioned control strains (Figure 2A). To confirm that it is the eosinophils that specifically promote antiviral responses, we transferred eosinophils isolated from the airways of B6-E2IL5tg mice (3-5 × 106 eosinophils/mouse) into the airways of cytokine-enriched, eosinophil-deficient B6-E2IL5tg-ΔdblGATA control counterparts immediately prior to inoculation with PVM. A significant percentage of eosinophils remained in the lungs of recipient mice and were detected 4 days after transfer (8 ± 4% eosinophils in recipients vs 0% in controls; Figure 2B). Eosinophil transfer into eosinophil-deficient recipient mice led to a significant reduction in virus recovery compared with recipient mice that received diluent only (Figure 2C). Finally, and remarkably, eosinophil-enriched B6-E2IL5tg mice were completely protected from an otherwise lethal inoculum of PVM, in sharp contrast to all other mouse strains evaluated (Figure 2D).
Eosinophils in the B6-E2IL5tg mouse airway inflammation model are antiviral and promote survival in response to a lethal respiratory virus infection. (A) Virus recovery t = 4 days after inoculation with PVM, determined as absolute copy number of virus SH gene per absolute copy number cellular GAPDH (PVMSH/GAPDH) in samples of total lung RNA; ***P < .001 (1-way ANOVA). (B) Cell differential at t = 4 days after adoptive transfer of eosinophils isolated from BAL fluid of B6-E2IL5tg mice (or vehicle alone) into the airways of recipient cytokine-enriched, eosinophil-deficient B6-E2IL5tg-ΔdblGATA mice immediately prior to inoculation with PVM. (C) Virus recovery (PVMSH/GAPDH from experiment described in (B); **P < .03 (Mann-Whitney, 2-tailed). (D) Survival of mice inoculated on day 0 with PVM, n = 5 to 10 mice per group; ***P < .001 (log-rank).
Eosinophils in the B6-E2IL5tg mouse airway inflammation model are antiviral and promote survival in response to a lethal respiratory virus infection. (A) Virus recovery t = 4 days after inoculation with PVM, determined as absolute copy number of virus SH gene per absolute copy number cellular GAPDH (PVMSH/GAPDH) in samples of total lung RNA; ***P < .001 (1-way ANOVA). (B) Cell differential at t = 4 days after adoptive transfer of eosinophils isolated from BAL fluid of B6-E2IL5tg mice (or vehicle alone) into the airways of recipient cytokine-enriched, eosinophil-deficient B6-E2IL5tg-ΔdblGATA mice immediately prior to inoculation with PVM. (C) Virus recovery (PVMSH/GAPDH from experiment described in (B); **P < .03 (Mann-Whitney, 2-tailed). (D) Survival of mice inoculated on day 0 with PVM, n = 5 to 10 mice per group; ***P < .001 (log-rank).
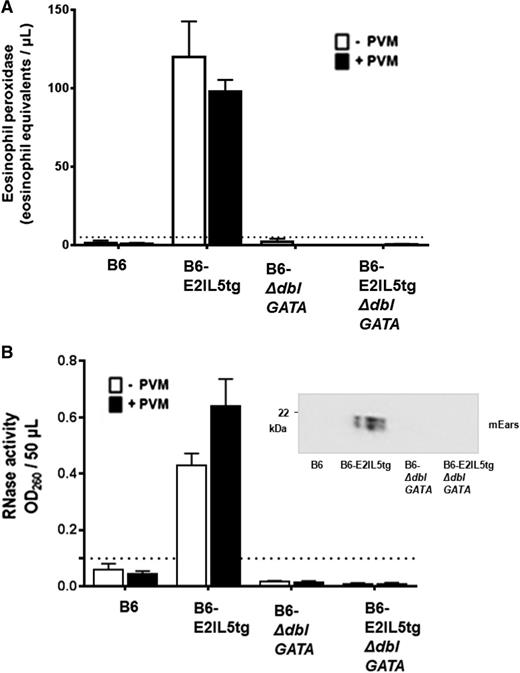
In addition to ongoing eosinophil recruitment, constitutive eosinophil degranulation in situ is a unique feature of this mouse asthma model. In contrast to what is observed in human asthma, in which eosinophil degranulation is prominent, mouse eosinophils do not degranulate readily in in vivo models of allergic sensitization and challenge.27-31 mEPX (Figure 3A) and mEars (Figure 3B) were detected in the airways (ie, in BAL fluid) in both virus-naïve and virus-infected B6-E2IL5tg mice; levels of both mEPX and RNase activity were below detectable limits in virus-naïve and virus-infected B6, B6-ΔdblGATA, and B6-E2IL5tg-ΔdblGATA controls. As such, eosinophils recruited to the lungs in the B6-E2IL5tg mouse asthma model are constitutively activated to the extent that virus challenge is not capable of eliciting further release of either granule protein.
Airway eosinophils in the B6-E2IL5tg mouse model are constitutively degranulating. (A) Immunoreactive mEPX was detected in BAL fluid from virus-naïve B6-E2IL5tg and PVM-infected B6-E2IL5tg mice only; n = 4 to 8 mice per group; ***P < .0001 vs all other conditions (2-way ANOVA); there is no statistically significant difference between the mEPX levels detected from virus naïve and virus-infected B6-E2IL5tg mice. (B) Ribonuclease (RNase) activity was detected in BAL fluid from virus-naïve and B6-E2IL5tg and PVM-infected B6-E2IL5tg mice only; n = 4 to 8 mice per group; ***P < .0001 vs all other conditions; there is no statistically significant difference between the RNase activities detected from virus naïve and virus-infected B6-E2IL5tg mice. (Inset) Western blot of BAL fluid from PVM-infected mice probed with rabbit polyclonal antisera against mEars. In both (A) and (B), dotted lines indicate lower limits of detection of each assay.
Airway eosinophils in the B6-E2IL5tg mouse model are constitutively degranulating. (A) Immunoreactive mEPX was detected in BAL fluid from virus-naïve B6-E2IL5tg and PVM-infected B6-E2IL5tg mice only; n = 4 to 8 mice per group; ***P < .0001 vs all other conditions (2-way ANOVA); there is no statistically significant difference between the mEPX levels detected from virus naïve and virus-infected B6-E2IL5tg mice. (B) Ribonuclease (RNase) activity was detected in BAL fluid from virus-naïve and B6-E2IL5tg and PVM-infected B6-E2IL5tg mice only; n = 4 to 8 mice per group; ***P < .0001 vs all other conditions; there is no statistically significant difference between the RNase activities detected from virus naïve and virus-infected B6-E2IL5tg mice. (Inset) Western blot of BAL fluid from PVM-infected mice probed with rabbit polyclonal antisera against mEars. In both (A) and (B), dotted lines indicate lower limits of detection of each assay.
Eosinophils recruited in response to Af antigens are also antiviral and protect against lethal virus infection
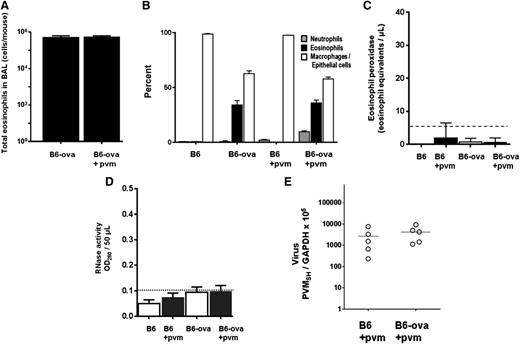
B6 mice were sensitized and challenged with Af extract via a standard protocol (Figure 4A) to determine whether eosinophils recruited to the airways in an antigen-dependent asthma model likewise displayed antiviral activity and to explore the role of degranulation in any antiviral activity observed. Af sensitization and challenge resulted in recruitment of eosinophils to the airways in numbers and percentages similar to those found in the cytokine-dependent B6-E2IL5tg model (Figure 4B-C).
PVM infection in the Af allergen-driven mouse airway inflammation model. (A) The Af sensitization, challenge, and PVM infection protocol used for these experiments. i.p., intraperitoneal inoculation; i.n., intranasal inoculation. The protocol for ova sensitization and challenge included i.p. inoculations on days 32 and 18, with all else remaining as shown. (B) Total eosinophils in BAL fluid from Af-sensitized and -challenged, virus-naïve, and PVM-infected B6 mice at day 4 after virus inoculation. (C) Cell differentials from mice in (B), as well as controls that were Af sensitized but not Af challenged. (D-E) Microscopic histology of lung tissue from mice that were Af sensitized and challenged and PVM infected (day 6 after PVM inoculation; see [A] for protocol); original magnifications ×10 and ×40, respectively. (F) Microscopic histology of lung tissue from mice that were Af sensitized but not Af challenged prior to PVM infection (day 6 after PVM inoculation); original magnification ×10.
PVM infection in the Af allergen-driven mouse airway inflammation model. (A) The Af sensitization, challenge, and PVM infection protocol used for these experiments. i.p., intraperitoneal inoculation; i.n., intranasal inoculation. The protocol for ova sensitization and challenge included i.p. inoculations on days 32 and 18, with all else remaining as shown. (B) Total eosinophils in BAL fluid from Af-sensitized and -challenged, virus-naïve, and PVM-infected B6 mice at day 4 after virus inoculation. (C) Cell differentials from mice in (B), as well as controls that were Af sensitized but not Af challenged. (D-E) Microscopic histology of lung tissue from mice that were Af sensitized and challenged and PVM infected (day 6 after PVM inoculation; see [A] for protocol); original magnifications ×10 and ×40, respectively. (F) Microscopic histology of lung tissue from mice that were Af sensitized but not Af challenged prior to PVM infection (day 6 after PVM inoculation); original magnification ×10.
Eosinophils recruited in response to Af antigens are not constitutively activated but degranulate in response to PVM
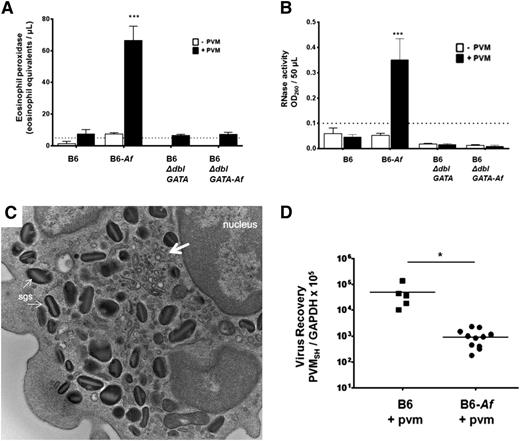
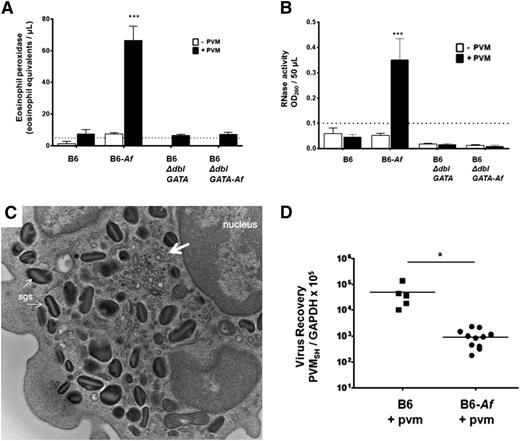
Allergen sensitization and challenge protocols have been used widely to model asthma in rodent species,32 ,33 although eosinophil degranulation in the airways is typically minimal.27-30 We reproduced this finding here. Although eosinophils were recruited in response to Af sensitization and challenge (Figure 4B-C), we detected no immunoreactive mEPX or RNase activity in the airways of these mice prior to virus infection. Eosinophils are found in clusters circumscribing the bronchioles in the lung tissue of Af-sensitized and -challenged, PVM-infected mice (Figure 1D-E), which are not detected in the lungs of PVM-infected mice and control mice (Figure 4F). However, following inoculation with PVM, elevated levels of immunoreactive mEPX (Figure 5A) and RNase activity (Figure 5B) were detected in the BAL fluid. No mEPX or RNase activity above background levels was detected in any of the controls. An electron micrograph of an eosinophil from the airways of an Af-sensitized and -challenged, PVM-infected mouse is shown in Figure 5C. Interestingly, we observe minimal emptying of the granules, and no evidence of eosinophil necrosis, cytolysis, and or granule extrusion. As such, the most likely mechanism to explain these observations is piecemeal degranulation34 ; at the arrows are subcellular structures with morphology consistent with eosinophil sombrero vesicles35 that may play a critical role in the directed secretion of these mediators. Interestingly, similar to, but somewhat less dramatic than, what was observed in the B6-E2IL5tg mice, a 1.6 log decrease in virus recovery was observed in mice that had been sensitized and challenged with Af antigens prior to PVM infection compared with controls (Figure 5D).
Eosinophils recruited in response to Af antigens are activated and degranulate in response to PVM and promote antiviral activity. (A) mEPX was detected in BAL fluid from Af-sensitized and Af-challenged, PVM-infected B6 wild-type mice only; n = 4 to 8 mice per group; ***P < .0001 vs Af-sensitized and Af-challenged, but not PVM-infected, group, as well as all other conditions. (B) RNase activity was detected in BAL fluid from Af-sensitized and Af-challenged, PVM-infected wild-type B6 mice only; n = 4 to 8 mice per group; ***P < .0001 vs Af-sensitized and Af-challenged, but not PVM-infected, group, as well as all other conditions. (C) Transmission electron micrograph of an eosinophil isolated from the airways of an Af-sensitized and Af-challenged, PVM-infected wild-type B6 mouse. Indicated in the image are specific granules and vesiculotubular structures34 analogous to those described in human eosinophils (at the large arrow); original magnification ×23 000. (D) Virus recovery from Af-sensitized and -challenged and control B6 wild-type or B6 eosinophil–deficient ΔdblGATA mice at 4 days after inoculation with PVM (see Figure 4A for timeline).
Eosinophils recruited in response to Af antigens are activated and degranulate in response to PVM and promote antiviral activity. (A) mEPX was detected in BAL fluid from Af-sensitized and Af-challenged, PVM-infected B6 wild-type mice only; n = 4 to 8 mice per group; ***P < .0001 vs Af-sensitized and Af-challenged, but not PVM-infected, group, as well as all other conditions. (B) RNase activity was detected in BAL fluid from Af-sensitized and Af-challenged, PVM-infected wild-type B6 mice only; n = 4 to 8 mice per group; ***P < .0001 vs Af-sensitized and Af-challenged, but not PVM-infected, group, as well as all other conditions. (C) Transmission electron micrograph of an eosinophil isolated from the airways of an Af-sensitized and Af-challenged, PVM-infected wild-type B6 mouse. Indicated in the image are specific granules and vesiculotubular structures34 analogous to those described in human eosinophils (at the large arrow); original magnification ×23 000. (D) Virus recovery from Af-sensitized and -challenged and control B6 wild-type or B6 eosinophil–deficient ΔdblGATA mice at 4 days after inoculation with PVM (see Figure 4A for timeline).
Eosinophils recruited in response to ova do not degranulate and do not promote antiviral activity
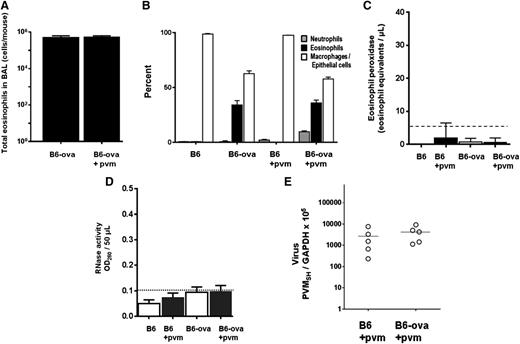
B6 mice were sensitized and challenged with ova via a slight modification of the standard protocol (“Methods” and Figure 4A). Similar to the response to Af antigens, eosinophils were prominent in the airways in response to ova sensitization and challenge (0.6 × 106 cells/mouse; Figure 6A-B). However, in stark contrast to what was observed in response to Af sensitization and challenge, we detect little to no degranulation under any circumstances in mice sensitized and challenged with ova, even in response to virus infection. No mEPX (Figure 6C) or any RNase activity (Figure 6D) was detected over background levels, and there was no differential virus recovery (Figure 6E).
Eosinophils recruited in response to ova sensitization and challenge do not degranulate in response to PVM infection and do not support antiviral activity. (A) Total eosinophils in BAL fluid from ova-sensitized and ova-challenged, both virus-naïve and PVM-infected B6 mice. (B) Cell differentials from mice in A, as well as controls that were ova sensitized and PBS challenged. (C) No immunoreactive mEPX was detected in any BAL fluids from this group; n = 4 to 6 mice per time point. (D) No RNase activity was detected in any BAL fluids from this group; n = 4 to 6 mice per time point. (E) Virus recovery from ova-sensitized and ova- or PBS-challenged wild-type B6 mice (Figure 4A).
Eosinophils recruited in response to ova sensitization and challenge do not degranulate in response to PVM infection and do not support antiviral activity. (A) Total eosinophils in BAL fluid from ova-sensitized and ova-challenged, both virus-naïve and PVM-infected B6 mice. (B) Cell differentials from mice in A, as well as controls that were ova sensitized and PBS challenged. (C) No immunoreactive mEPX was detected in any BAL fluids from this group; n = 4 to 6 mice per time point. (D) No RNase activity was detected in any BAL fluids from this group; n = 4 to 6 mice per time point. (E) Virus recovery from ova-sensitized and ova- or PBS-challenged wild-type B6 mice (Figure 4A).
Af sensitization and challenge: time course and survival
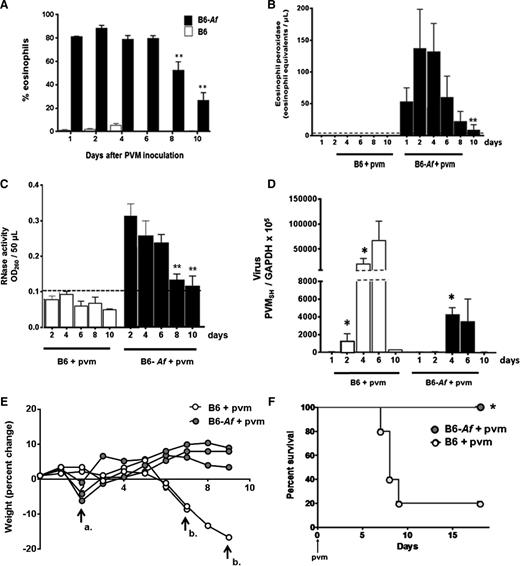
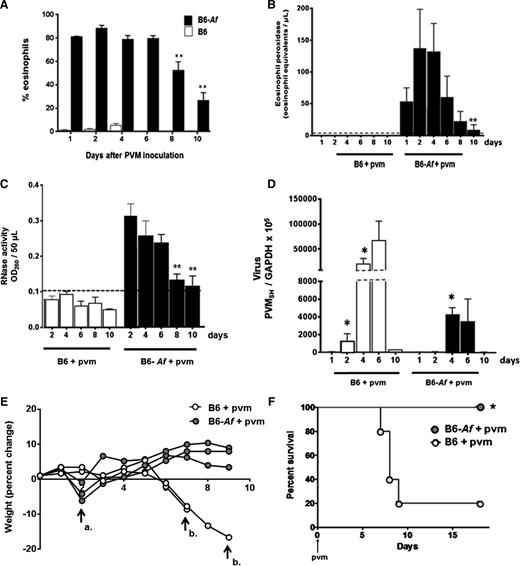
We returned to the Af sensitization and challenge protocol to examine eosinophil recruitment, degranulation, virus recovery, and clinical impact over time in B6 wild-type mice. Referring back to the time course shown in Figure 4A, mice are sensitized and challenged with Af as shown and inoculated with PVM on day 0. As shown in Figure 7A, eosinophils are detected in the airways of Af-sensitized and -challenged mice, and they remain prominent from day 1 through day 10 of virus infection, although the population declines beginning at day 8. The eosinophil granule protein mEPX (Figure 7B) and RNase activity (Figure 7C) follow the same time course. Virus recovery is documented in Figure 7D. Virus can be detected above background at day 2 in control mice; recovery peaks at day 6 and is cleared by day 10. In contrast, virus cannot be detected over background at day 2 in Af-sensitized and -challenged mice; recoveries at days 4 and 6 are ∼1 log less than in the control mice (Figure 4D). Virus recovery is near or beneath detectable limits in both control and Af-sensitized and -challenged mice by day 10.
Eosinophil degranulation products persist in the airways of mice subjected to Af sensitization and challenge and are associated with protection from the lethal sequelae of PVM infection. (A) Wild-type B6 mice subjected to Af sensitization and Af or PBS challenge prior to PVM infection on day 0 (see Figure 4A for protocol) and percent eosinophils were evaluated in BAL fluid as shown; P < .0001 for B6-Af vs B6 at all points (not shown on figure); **P < .03 vs percentage eosinophils at day 1. (B) Immunoreactive mEPX detected in mice that were Af sensitized and Af challenged prior to PVM infection only (B6-Af+pvm); P < .0001 for B6-Af vs B6 at all points (not shown on figure), **P < .03 compared with peak levels detected at day 2. (C) RNase activity detected in BAL fluid of mice as described in A; P < .0001 for B6-Af vs B6 at all points (not shown on figure); **P < .03 compared with peak levels detected at day 2. (D) Virus recovery from lung tissue of mice that were Af sensitized and PBS challenged vs Af sensitized and Af challenged prior to PVM infection; *P < .05 compared with recovery at day 1. (E) Percent weight change of mice subjected to Af sensitization and PBS or Af challenge prior to PVM infection. Arrow a. denotes transient weight loss at day 2 experienced by the Af-sensitized and Af-challenged mice, and arrow b. designates loss of PVM-infected, Af-sensitized, PBS-challenged mice at days 7 and 9. (F) Survival of Af-sensitized and PBS- or Af-challenged mice prior to PVM infection on day 0; n = 5 mice per group; *P < .03 (log-rank).
Eosinophil degranulation products persist in the airways of mice subjected to Af sensitization and challenge and are associated with protection from the lethal sequelae of PVM infection. (A) Wild-type B6 mice subjected to Af sensitization and Af or PBS challenge prior to PVM infection on day 0 (see Figure 4A for protocol) and percent eosinophils were evaluated in BAL fluid as shown; P < .0001 for B6-Af vs B6 at all points (not shown on figure); **P < .03 vs percentage eosinophils at day 1. (B) Immunoreactive mEPX detected in mice that were Af sensitized and Af challenged prior to PVM infection only (B6-Af+pvm); P < .0001 for B6-Af vs B6 at all points (not shown on figure), **P < .03 compared with peak levels detected at day 2. (C) RNase activity detected in BAL fluid of mice as described in A; P < .0001 for B6-Af vs B6 at all points (not shown on figure); **P < .03 compared with peak levels detected at day 2. (D) Virus recovery from lung tissue of mice that were Af sensitized and PBS challenged vs Af sensitized and Af challenged prior to PVM infection; *P < .05 compared with recovery at day 1. (E) Percent weight change of mice subjected to Af sensitization and PBS or Af challenge prior to PVM infection. Arrow a. denotes transient weight loss at day 2 experienced by the Af-sensitized and Af-challenged mice, and arrow b. designates loss of PVM-infected, Af-sensitized, PBS-challenged mice at days 7 and 9. (F) Survival of Af-sensitized and PBS- or Af-challenged mice prior to PVM infection on day 0; n = 5 mice per group; *P < .03 (log-rank).
Eosinophil recruitment and degranulation was associated with percent weight change and survival in response to acute PVM infection. In Figure 7E, control mice underwent a period of rapid weight loss prior to demise, as is typical for a lethal PVM strain J3666 infection. Interestingly, mice that were Af sensitized and challenged underwent a transient and minimal (1-5% total body weight) weight loss at day 2 after PVM infection. These mice then recovered and went on to survive throughout with no further significant weight loss detected. The weight loss at day 2 may represent a response to eosinophil activation and release of granule products (Figure 7B), although this remains to be fully explored. Finally, as was observed with eosinophil-enriched B6-E2IL5tg mice, Af sensitization and challenge resulted in full protection from the otherwise lethal virus inoculum (Figure 7F).
Discussion
In this study, we established acute respiratory virus infection with PVM in 3 distinct mouse models of Th2 cytokine–driven asthmatic inflammation, specifically cytokine-driven inflammation in B6-E2IL5tg mice and allergen-driven inflammation via senstization and challenge with either ova or Af antigens. Among our results, we found that eosinophils recruited to the airways of otherwise naïve mice in response to Af, but not ova, sensitization and challenge degranulate specifically in response to PVM infection.
Eosinophil degranulation is not a routine feature of allergen sensitization and challenge airway inflammation models in the mouse.27-30 This observation led Lee and Lee31 to consider the possibility that degranulation itself might be a vestigial function of this granulocyte lineage. Our results suggest an alternative explanation for this observation, specifically, that allergen sensitization and challenge of otherwise naïve mice is simply not a sufficient stimulus to elicit degranulation. We show here that eosinophils recruited to the airways in response to Af sensitization and challenge do not degranulate on their own, but do so in response to subsequent activation with PVM, a natural mouse pathogen that undergoes robust replication in alveolar macrophages and bronchiolar epithelial cells and elicits production of proinflammatory cytokines.15-17
Interestingly, and for reasons as yet unclear, eosinophils recruited in response to ova sensitization and challenge do not respond in an analogous fashion to PVM. Although both ova and Af promote eosinophil recruitment when administered via the standard sensitization and challenge protocol, ova is otherwise inert (save for potential contamination with lipopolysaccharide36 ), whereas Af extract is a complex mix of antigens including those that can signal via Toll-like receptors (TLRs) 2 and 4 expressed on bone marrow dendritic cells, macrophages, lung epithelial cells,37-39 and conceivably eosinophils.40 Zimmermann et al41 compared the ova and Af sensitization challenge models directly to one another via DNA microarray; among the 236 transcripts unique to the Af sensitization and challenge model are those encoding several proinflammatory cytokines, including IL-1β, IL-1α, CCL3, and most notably, IL-6, which has already been shown to have an impact on virus-eosinophil interactions.42 The precise mechanisms via which Af (but not ova) primes eosinophils to respond to virus infection remain to be explored. Of note, Davoine et al43 reported that human eosinophils interact with parainfluenza virus and release peroxidase only when in coculture with antigen presenting cells and CD4+T lymphocytes, findings that would also be consistent with a more complex mechanism.
Likewise, we show here for the first time that eosinophil activation is associated with a reduction in virus load and with protection against the lethal sequelae of PVM infection. As we have shown in our previous work with the PVM infection model, absolute virus recovery is only 1 factor contributing to a lethal outcome26,44,45 ; virus-mediated activation of eosinophils might also result in release cytokines, chemokines, reactive oxygen intermediates, and lipid mediators that could modulate the inflammatory impact of PVM infection. Indeed, we have preliminary data documenting the presence of high concentrations of CCL2, CCL3, and CXCL10 in the airways of B6-E2IL5tg mice but not eosinophil-deficient B6-E2IL5tg ΔdblGATA mice (C.M.P. and H.F.R., unpublished data, 2013), although additional experiments will be needed to confirm that these mediators are derived directly from eosinophils. Ongoing explorations will focus on the mechanisms of eosinophil-mediated antiviral host defense and will include experiments that document the role(s) of unique cationic granule proteins using specific antibodies and gene-deleted mice.29,30
Despite the substantial reduction in virus recoveries observed in vivo (Figures 2A and 5D), we have been unable to recapitulate similar findings when isolating constitutively degranulating eosinophils from B6-E2IL5tg mice and incubating them ex vivo with PVM virions (C.M.P. and H.F.R., unpublished data, 2013); these experiments suggest that eosinophil degranulation products may not be targeting virions directly. Among the indirect mechanisms under consideration, eosinophil secretory mediators may render target cells resistant to infection or may have a means to target and destroy virus-infected cells. As an example of the latter mechanism, in our earlier study of the human eosinophil granule protein, EDN/RNase 2, and its interactions with RSV in vitro, we initially proposed a direct, lytic interaction between the virus and EDN,11 but later considered the possibility that EDN might “hijack” its way into a target cell via a shared cell receptor or extracellular interaction with the virion.8 It is certainly possible that the antiviral mechanism in vivo may be even more complex, as eosinophils have extensive immunomodulatory functions.7,46,47 As 1 example, PVM-mediated release of granule proteins from eosinophils may result in leukocyte recruitment and activation at later time points; for example, the granule ribonuclease mEar 2 has been characterized as a chemoattractant for mouse dendritic cells in vivo.48 The complete characterization of virus-mediated degranulation and responses to this event in vivo is under further study in our laboratory.
In summary, we showed that activated eosinophils promote a profound antiviral state in 2 distinct models of Th2-driven inflammation. This is the first time that eosinophils have been shown to have a unique physiologic, protective role in the setting of a severe respiratory virus infection. Thus, although eosinophil activation and degranulation are generally interpreted as purely detrimental, pathologic events, our work provides the first evidence that degranulating airway eosinophils, as components of a pathophysiologic Th2-polarized inflammatory response, serve to promote survival in response to a lethal virus infection. As such, the more profound eosinophil depletion strategies under development for various diseases including asthma49 may merit caution in light of the beneficial role of these cells described here.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors present this manuscript as a tribute to our colleague and esteemed scientist, the late Dr Redwan Moqbel, who pioneered our current understanding of eosinophil degranulation. The authors thank Dr Nariman Balenga for advice on A fumigatus protocols and Dr Kirk Druey for ongoing advice and in-depth and critical comments on the project and multiple drafts of the manuscript. The authors also thank Dr Alfonso Gozalo and the staff of the National Institutes of Health, National Heart, Lung, and Blood Institute 14BS animal facility for the care of the mice used in these experiments.
This study was supported by National Institutes of Health, National Institutes of Allergy and Infectious Diseases, Division of Intramural Research grants AI000941 to AI000943 (H.F.R.); funds from the Mayo Foundation (N.A.L.); National Institutes of Health, National Heart, Lung, and Blood Institute grants R01-HL058723 (N.A.L.) and R01-HL065228 (J.J.L.); and a National Institutes of Health, National Center for Research Resources grant RR0109709 (J.J.L.).
Authorship
Contribution: C.M.P. designed and performed experiments, analyzed data, prepared figures, and assisted with first and subsequent drafts of the manuscript; K.D.D. analyzed data and assisted with manuscript revisions; S.I.O. provided crucial reagents and assisted with manuscript revisions; J.L.L. performed experiments contributing toward the revision of the manuscript; E.R.F. performed the electron microscopy; J.J.L. provided advice on experimental design, crucial reagents, and assisted with manuscript revisions; N.A.L. provided crucial reagents and assisted with manuscript revisions; J.B.D. provided overall direction and assistance with manuscript revisions; and H.F.R. designed experiments, analyzed data, edited figures, and prepared the first and subsequent drafts of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Helene F. Rosenberg, Building 10, Room 11C215, MSC-1883, IIS/LAD/NIAID/NIH, 9000 Rockville Pike, Bethesda, MD 20892; e-mail: hrosenberg@niaid.nih.gov.

![Figure 1. PVM infection in the cytokine-driven B6-E2IL5tg mouse model of airway inflammation. (A) Total eosinophils in BAL fluid from PVM-infected C57BL/6 (B6) wild-type, B6-eotaxin-2/IL-5 double transgenic (B6-E2IL5tg), eosinophil-deficient B6-ΔdblGATA, and cytokine-enriched eosinophil-deficient B6-E2IL5tg-ΔdblGATA mice; t = 4 days after PVM inoculation; n = 4 to 6 mice per time point; ***P < .0001 (1-way analysis of variance [ANOVA]). (B) Cell differentials of mice described in A. (C-D) Microscopic histology of lung tissue from PVM-infected B6-E2IL5tg mice; original magnifications ×10 and ×40, respectively. (E-F) Microscopic histology of lung tissue from PVM-infected B6-E2IL5tg-ΔdblGATA mice; original magnifications ×10 and ×40, respectively; area within box in (E) magnified as shown in (F).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/123/5/10.1182_blood-2013-05-502443/4/m_743f1.jpeg?Expires=1770232878&Signature=ZjE6KmSg5Qt4GL6P4xY27k4reRy51ExbZnTTGwIMswaTPxBa79ylWnY~naO-wehuJrfDVOVhz6Bn7cQcIaF0UhlsR3KyiucAo1JzTHB7bOykF6ip7jH19~o7M7LQ92E7i~UeFytVDkqhCi2QVem0U-6dMjdiSVxiqjtZoBcEZtkYt-8MaRTGgKaA2gBqKgAlMp-zTpLMs9ZnaCW690WdqKcQWw6ThSc78wL5Ypqqv93Vqwa8hDN7BZZFXZe6DCQ7xbfKfbzJe27H7gYnaxem0x8gJbvsZIMTLGagcaxOVj9zvZ0s-bH0W-TwuA8vH6XkUe07JKJkE7e~QnMfWf0hwA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. PVM infection in the Af allergen-driven mouse airway inflammation model. (A) The Af sensitization, challenge, and PVM infection protocol used for these experiments. i.p., intraperitoneal inoculation; i.n., intranasal inoculation. The protocol for ova sensitization and challenge included i.p. inoculations on days 32 and 18, with all else remaining as shown. (B) Total eosinophils in BAL fluid from Af-sensitized and -challenged, virus-naïve, and PVM-infected B6 mice at day 4 after virus inoculation. (C) Cell differentials from mice in (B), as well as controls that were Af sensitized but not Af challenged. (D-E) Microscopic histology of lung tissue from mice that were Af sensitized and challenged and PVM infected (day 6 after PVM inoculation; see [A] for protocol); original magnifications ×10 and ×40, respectively. (F) Microscopic histology of lung tissue from mice that were Af sensitized but not Af challenged prior to PVM infection (day 6 after PVM inoculation); original magnification ×10.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/123/5/10.1182_blood-2013-05-502443/4/m_743f4.jpeg?Expires=1770232878&Signature=tC8sr~u~uol1406nZFNgVtqVEbhGooEtA5MCOGknoKBKcakCfl~-km~OD~Lrgh8lHNBxb-I2nNpSZ8fMgqkSBTZGJzpKimQKCW0s~fraZt8KCFEVV0CXTbtB81QCf2q6VVX6FT8ruQZfEYgegz~0cp~-RD-6tmTViCQp8LfHpXHCgVQZJS6eg-WJ4e4gedztVq~kVS5uDtFieSBQlJtddOYGSi5pBNayzxtUJir7oUsFa-f~j9uhzJwaQ5x9cOf2r-ptWwIEUxYRurhAVIHFl-f4PhOJT89ZJ24CfoxbpvOVhTMlH44lVN3ka9SHKYMm48oYMsd5-dS4VSPD9gpa0w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. PVM infection in the cytokine-driven B6-E2IL5tg mouse model of airway inflammation. (A) Total eosinophils in BAL fluid from PVM-infected C57BL/6 (B6) wild-type, B6-eotaxin-2/IL-5 double transgenic (B6-E2IL5tg), eosinophil-deficient B6-ΔdblGATA, and cytokine-enriched eosinophil-deficient B6-E2IL5tg-ΔdblGATA mice; t = 4 days after PVM inoculation; n = 4 to 6 mice per time point; ***P < .0001 (1-way analysis of variance [ANOVA]). (B) Cell differentials of mice described in A. (C-D) Microscopic histology of lung tissue from PVM-infected B6-E2IL5tg mice; original magnifications ×10 and ×40, respectively. (E-F) Microscopic histology of lung tissue from PVM-infected B6-E2IL5tg-ΔdblGATA mice; original magnifications ×10 and ×40, respectively; area within box in (E) magnified as shown in (F).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/123/5/10.1182_blood-2013-05-502443/4/m_743f1.jpeg?Expires=1770320316&Signature=XPQRyz49wbELSGJTDl59~VHcJABxC78RNDIrApl-dZXYmardot9wTNKvMYdpF4YJdE9b3c~9ES38hQwGJK2sRHL6FETyFqDV42UiE3Sa23y8pNK1SabsSJxLGCoLuiEV6wL~uH-OZA5nvlaiP1Ib-0EqINeOqJmgnUsZbS8MNtAd5lET-l~IDsmrlRLiac9ZFKZg4pu9nyHlvhwOLBxlymihWk13gHjL2MP6-vtnAvTMhJkNbYQj7995fz1Gnyo9vI4cu~3xlWvI7ufN8Yjbj0St9OWQpR9bxF6aJp8fw~quUC7CZXDTG8f16xbVUsdUADSSfoleXpT-epudJu~uRw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. PVM infection in the Af allergen-driven mouse airway inflammation model. (A) The Af sensitization, challenge, and PVM infection protocol used for these experiments. i.p., intraperitoneal inoculation; i.n., intranasal inoculation. The protocol for ova sensitization and challenge included i.p. inoculations on days 32 and 18, with all else remaining as shown. (B) Total eosinophils in BAL fluid from Af-sensitized and -challenged, virus-naïve, and PVM-infected B6 mice at day 4 after virus inoculation. (C) Cell differentials from mice in (B), as well as controls that were Af sensitized but not Af challenged. (D-E) Microscopic histology of lung tissue from mice that were Af sensitized and challenged and PVM infected (day 6 after PVM inoculation; see [A] for protocol); original magnifications ×10 and ×40, respectively. (F) Microscopic histology of lung tissue from mice that were Af sensitized but not Af challenged prior to PVM infection (day 6 after PVM inoculation); original magnification ×10.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/123/5/10.1182_blood-2013-05-502443/4/m_743f4.jpeg?Expires=1770320317&Signature=vU5el~wkW9b8OkuYM5rnIrO0Z0N9eGA3lZB42gmxrrLkmEuJz3t4IbOEVj1scLUOd7alVlc0CAU6a2UOgHqT2sHtugQWbVrnhQJ3x-FAkQwJA0FN~ETEURSJzJa7VockR8xfbLcv8PLJLpGt81l8QgWaT9OSRFA6cKXLDQCM0L1n1lV28YWa9RHZAHjglgk5-vhNIYR~mexgODBV47-i0MH2qFeTnEIxZu6lJ477cDjm1GCzakXzlgiUEg8K~Nul4HWWD32AJWH8PRiE-xpkvG4Qu2cLKakJZxCMajCqjJlo37ASTnB0k8kALZJMSKUD9kQMG8z-kd33VynkMx3Oqw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)