Key Points
Erythropoietin therapy can be effective to hasten erythroid recovery and reduce transfusion requirements after allogeneic HCT.
Abstract
We conducted a prospective randomized trial to assess hemoglobin (Hb) response to recombinant human erythropoietin (rhEPO) therapy after hematopoietic cell transplantation (HCT). Patients (N = 131) were randomized (1:1) between no treatment (control arm) or erythropoietin at 500 U/kg per week (EPO arm). Patients were also stratified into 3 cohorts: patients undergoing myeloablative HCT with rhEPO to start on day (D)28, patients given nonmyeloablative HCT (NMHCT) with rhEPO to start on D28, and patients also given NMHCT but with rhEPO to start on D0. The proportion of complete correctors (ie, Hb ≥13 g/dL) before D126 posttransplant was 8.1% in the control arm (median not reached) and 63.1% in the EPO arm (median, 90 days) (P < .001). Hb levels were higher and transfusion requirements decreased (P < .001) in the EPO arm, but not during the first month in the nonmyeloablative cohort starting rhEPO on D0. There was no difference in rates of thromboembolic events or other complications between the 2 arms. This is the first randomized trial to demonstrate that rhEPO therapy hastens erythroid recovery and decreases transfusion requirements when started one month after allogeneic HCT. There was no benefit to start rhEPO earlier after NMHCT.
Introduction
Erythropoiesis is regulated by erythropoietin (Epo), with serum Epo levels increasing exponentially when an anemia develops.1,2 After high-dose chemotherapy, serum Epo rapidly increases to disproportionately high levels for 1 to 3 weeks, with a peak in the week after conditioning.3,4 However, after myeloablative (MA) allogeneic hematopoietic cell transplantation (HCT), Epo response to anemia then becomes impaired: Epo levels increase in absolute terms but not enough for the degree of anemia, resulting in inappropriately low Epo levels and prolonged anemia.4 However, serum Epo levels remain adequate throughout the posttransplant course after nonmyeloablative HCT (NMHCT).5
Epo expands erythropoiesis mainly by preventing apoptosis of erythroid progenitors and proerythroblasts.6 Epo is therefore unlikely to increase red blood cell (RBC) production when endogenous Epo is elevated and progenitors are already surviving and differentiating. Previous trials of recombinant human Epo (rhEPO) after HCT have administered very high doses of intravenous (IV) rhEPO (usually >1000 U/kg per week) starting on day (D)1 and continuing for 1 to 2 months or until erythroid engraftment, and they have shown little benefit with a prohibitive cost.7-12 Therefore, soaking patients with huge doses of rhEPO, when the erythroid marrow has not developed enough erythroid precursors and endogenous Epo levels are appropriate or excessive for the degree of anemia, may not be ideal.
We previously showed in a pilot study that rhEPO could be very efficient when rhEPO therapy was started 35 days after MA allogeneic HCT at 500 U/kg per week.13 The hemoglobin (Hb) response rate was >90%. Moreover, the same weekly dose of rhEPO was as effective when given once weekly or in 3 divided doses.14
We also carried out a pilot trial of rhEPO therapy in patients undergoing NMHCT.15 A first group started rhEPO on D0 and a second started on D28 after transplantation. Compared with historical controls, Hb values were higher but transfusion requirements were decreased only in the first month in patients receiving rhEPO from D0. Therefore, rhEPO therapy also appeared to be efficient after NMHCT, but less so after conventional HCT. This prompted us to conduct a randomized trial to assess Hb response and transfusion requirements after allogeneic transplantation with or without rhEPO.
Patients and methods
Patients
Patients underwent allogeneic transplantation for malignant or nonmalignant diseases after conventional or nonmyeloablative conditioning. Patients were eligible if they did not have terminal organ failure, had sufficient iron stores (serum ferritin >100 µg/L), and had adequate marrow recovery (neutrophils >1 × 109/L and platelet transfusion independence). Patients who underwent 2 allogeneic HCT (for rejection or relapse) could be included twice and were considered as “separate subjects” for analyses. They received peripheral blood stem cell (PBSC) or marrow (not cord blood) transplantation and the graft was unmanipulated or T-cell depleted. Posttransplant immunosuppression was based on cyclosporine A or tacrolimus (± other agents). Exclusion criteria included HIV seropositivity; known allergy to rhEPO or IV iron; evidence of active hemorrhage, hemolysis, vitamin B12 or folate deficiency; or uncontrolled infection, arrhythmia, or hypertension. In these cases, inclusion into the protocol could be delayed up to 14 days if the problem was resolved. Patients with a Hb level >13 g/dL at treatment initiation were also excluded.
Between D21 and D28 posttransplant if they were scheduled to start rhEPO on D28, or before transplantation if they were scheduled to start on D0, patients signed informed consents and were randomized 1:1 between the control and EPO arms on the same day. There was no death or dropout between randomization and start of the study. Patients were stratified for the type of conditioning (MA vs non-MA) and for the start (D0 or D28) of rhEPO therapy for NMHCT. Hence, we had 3 cohorts: the first cohort included patients undergoing MA HCT with rhEPO to be started on D28, the second cohort patients given NMHCT with rhEPO to be started on D28, and the third cohort patients undergoing NMHCT with rhEPO to be started on D0. Based on our pilot studies in the MA13 and non-MA15 settings, we calculated that we needed 118 evaluable patients to have a 90% power to detect a significance level of 0.05, a difference in the primary end points of 75%, 50%, and 45% in the MA, non-MA D28, and nonmyeloablative D0 cohorts, respectively. The study started with the first 2 cohorts only, to which patients were assigned on the basis of their conditioning (MA vs non-MA), all starting rhEPO on D28. When the results of our pilot rhEPO trial after NMHCT became available,15 we added through an amendment the third cohort (NMHCT with rhEPO from D0) that was scheduled to start only when recruitment in cohort 2 (NMHCT with rhEPO from D28) was completed. Study procedures remained otherwise identical. Randomization was carried out by following up a computer-generated randomization list, unavailable to clinical investigators, for each cohort.
Patients (or his/her guardian if of minor age) signed an informed consent for the clinical study, as well as to collect and analyze blood samples, and the study was approved by the Ethics Committee of the University of Liège under number 2003/59. The study was conducted in accordance with the Declaration of Helsinki.
RhEPO therapy
RhEPO (Neorecormon, Roche) was administered subcutaneously at 500 U/kg once per week. RhEPO doses were never raised above 500 U/kg/week. The first dose was given on D28, except in the third cohort (D0). Once the target Hb level (13 g/dL) was attained, the dose of rhEPO was reduced by half. If the Hb increased to >14 g/dL, rhEPO was withheld and resumed at the dose of 250 U/kg per week when the Hb level decreased to <13 g/dL. If the Hb level decreased to <12 g/dL, the dose of rhEPO was again increased to 500 U/kg per week. Maximum doses of 16 × 500 U/kg per week were to be administered. Maintenance with the lowest possible dose of rhEPO was allowed after D126. Treatment was stopped in case of disease relapse.
In the EPO arm, if patients had evidence of functional iron deficiency (Tsat <20%), they received 300 mg IV iron saccharate (Venofer) on 2 consecutive visits at least one week apart. Iron therapy was repeated if functional iron deficiency persisted. Iron was omitted in patients with severe iron overload (serum ferritin >2500 µg/L without inflammation or liver necrosis). Control patients never received IV iron. One RBC unit was transfused if the Hb value was between 7.0 and 7.9 g/dL, and 2 units were transfused when it was <7 g/dL. Platelets were transfused if needed per the institution’s standards.
Laboratory analyses
Laboratory data were monitored weekly from the day of the transplant (D0) until D28, and then every other week. Complete blood counts, percentage and absolute reticulocyte counts, serum Epo levels, serum soluble transferrin receptor (sTfR) (a quantitative measure of total erythropoietic activity), serum iron, Tsat, and ferritin were measured as previously reported.5,13,14,16-18
Study end points
The primary end points were the median time to achieve a Hb level ≥13 g/dL and proportion of complete correctors (reaching Hb ≥13 g/dL) before D126 (ie, after 14 weeks of treatment). Secondary end points included median time to increase Hb by ≥2 g/dL; the proportion of responders (ie, patients increasing Hb by ≥2 g/dL) and correctors (ie, patients reaching Hb ≥12 g/dL) before D126; the proportion of patients requiring RBC transfusions and the total number of RBC transfusions between D28 and D126; the area under the curve (AUC) of Hb between D28 and D126 after transplantation; the mean Hb values on D42, D60, D80, D100, and D126; and the mean change in quality of life (QOL) measurements. QOL evaluations were carried out at baseline (D28 or D0 for the 3rd cohort) as well as on D30, D70, and D150 after rhEPO initiation based on the validated Functional Assessment of Cancer Therapy (FACT)-anemia questionnaire.19 All analyses were planned for the whole study population as well as for all 3 cohorts separately.
Statistical analyses
Median times from rhEPO initiation to events were assessed using cumulative incidence methods and compared by the log-rank test. These end points were also calculated from D28 posttransplant for control patients (or from D0 in the third cohort). Patients were censored at the time of graft rejection, severe autoimmune hemolysis, aplasia induced by chemotherapy or radiotherapy, and/or disease progression. For censored patients, the last Hb value before censure was carried forward. Numbers of transfusions, QOL scores, and Hb, reticulocyte, sTfR, Tsat, and ferritin levels were compared using the Mann-Whitney U test. AUCs of Hb were calculated from D28 to D126 post-HCT (14 weeks of treatment). Two-way analysis of variance was used to analyze the impact of treatment group and time point (D0 vs D30 vs D70 vs D150 post-rhEPO) on QOL scores. Correlations between QOL and Hb levels were assessed with the Spearman test. Results are presented as means and standard deviations or numbers and percentages. To assess relapse risks, univariate and multivariate Cox regression models (including disease risk index, treatment arm, and conditioning) were performed. Statistical analyses were carried out with Graphpad Prism (Graphpad Software, San Diego, CA) and SAS, 9.3 for Windows (SAS Institute, Cary, NC); some graphs were realized with S-Plus, 8.2.
Results
Patients
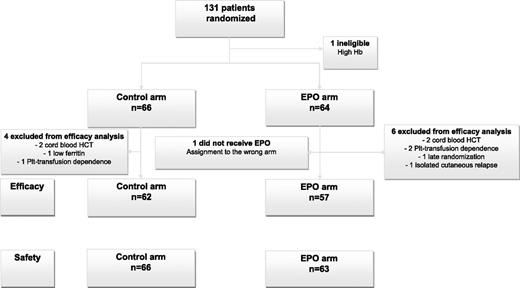
We included 131 patients between May 2003 and January 2008. Twelve patients were found to be ineligible: 4 in the control arm (cord blood HCT [n = 1]), platelet transfusion dependence (n = 1), ferritin <100 µg/L [n = 2]) and 8 in the EPO arm (cord blood HCT [n = 2], platelet transfusion dependence ]n = 2], relapse [n = 1], late randomization [n = 1], high baseline Hb [n = 1], assignment to the wrong arm [n = 1]) (Figure 1). Characteristics of the 119 patients evaluable for efficacy are detailed in Table 1. These characteristics were comparable between arms, except for cytomegalovirus (CMV) status (P = .0179). Graft composition and speed of engraftment (slower after MA conditioning) were not significantly different between the 2 arms overall and in the different cohorts (supplemental Table 1, available on the Blood Web site).
Treatments
No patient in the control arm received rhEPO. Treatment initiation was delayed by 1 week in 6 patients and by 2 weeks in 4 patients. The total number of rhEPO injections until D126 was 12.6 ± 4.6. The most usual dose was between 30 000 U and 40 000 U (1 injection of 30 000 U [0.6 mL] and 1 injection of 10 000 U [0.6 mL]) per week. One patient undergoing NMHCT with rhEPO started on D0 stopped on D61 (after 10 doses) because of inefficacy in the context of infection and major ABO mismatch. Moreover, 19 patients received maintenance therapy after D126.
Primary end points
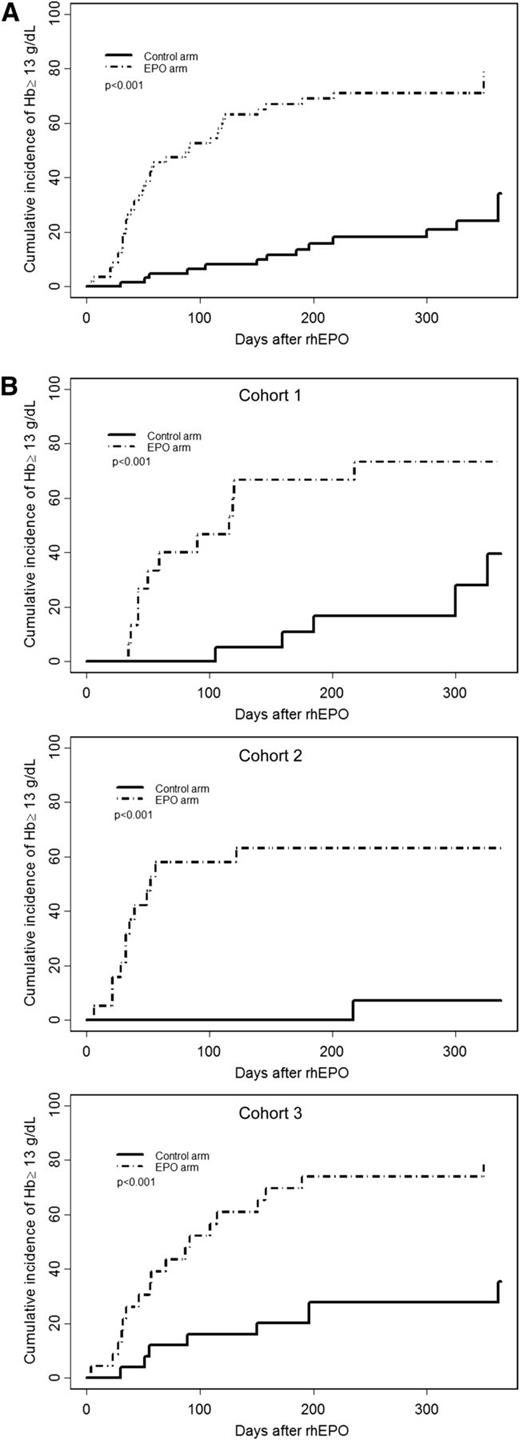
Median times to achieve a Hb value ≥13 g/dL were undetermined in the control arm and 90 days in the EPO arm (P < .001) (Figure 2A). The difference remained highly significant (P < .001) when only patients with baseline Hb values <10 g/dL were considered (post hoc analysis). Median times to reach a Hb value ≥13 g/dL in the control and EPO arms, respectively, were undetermined and 116 days after MA conditioning; not defined and 52 days after rhEPO started on D28 after NMHCT; and undefined and 31 days with rhEPO initiated on D0 (Figure 2B).
Cumulative incidence of complete response (Hb ≥13 g/dL) (primary end point) from the day of treatment initiation. (A) All conditionings together; (B) after MA conditioning (Cohort 1), after NMHCT with rhEPO started on D28 (Cohort 2), and after NMHCT when rhEPO initiated on D0 (Cohort 3). The control arm is indicated by a solid line, and the EPO arm by a dotted line.
Cumulative incidence of complete response (Hb ≥13 g/dL) (primary end point) from the day of treatment initiation. (A) All conditionings together; (B) after MA conditioning (Cohort 1), after NMHCT with rhEPO started on D28 (Cohort 2), and after NMHCT when rhEPO initiated on D0 (Cohort 3). The control arm is indicated by a solid line, and the EPO arm by a dotted line.
The proportions of complete correctors (achieving 13 g/dL) before D126 posttransplant were 8.1% in the control arm and 63.1% in the EPO arm (P < .001). After MA conditioning, complete correctors were 5.2% and 66.7%, respectively (P < .001). These proportions were 0% and 63.2%, respectively, when rhEPO was started on D28 (P < .001); and 16% and 60.9%, respectively, when rhEPO was started on D0 (P < .001) after NMHCT. The proportion of complete correctors was not significantly different among the 2 rhEPO cohorts after NMHCT.
Secondary end points
The proportions of responders (patients increasing their Hb value by ≥2 g/dL) were 25% in the control arm and 73.2% in the EPO arm, and median times to response were undefined and 42 days, respectively (P < .001). The proportions of correctors (patients reaching 12 g/dL) were 22.5% in the control arm and 71.9% in the EPO arm, and median times to correction were undetermined and 53 days, respectively (P < .001). The difference remained highly significant (P < .001) when only patients with baseline Hb values <10 g/dL were considered (post hoc analysis). We also analyzed these end points in each cohort separately: median times to response were shorter and proportions of responders or correctors were higher in patients receiving EPO compared with controls (Table 2).
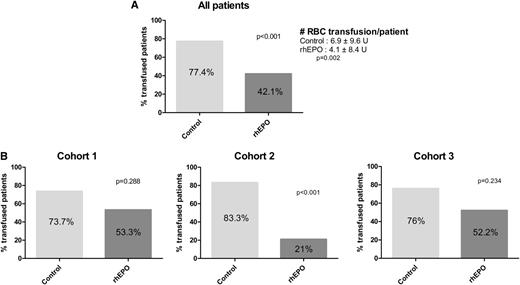
The proportions of patients requiring RBC transfusions, as well as the number of units transfused per patient, were higher in the control arm compared with the EPO arm (Figure 3A). The difference remained highly significant (P < .001 for proportions of transfused patients and P = .017 for the number of transfused units) when only patients with baseline Hb values <10 g/dL were considered (post hoc analysis). Analyzing the 3 cohorts separately, we observed only a trend toward decreased transfusion rates with rhEPO, except when rhEPO was started on D28 after NMHCT, when the difference was significant (Figure 3B). We also asked whether there was any benefit to start rhEPO earlier (D0 rather than D28) after NMHCT (post hoc analysis). There was no significant difference between the 2 rhEPO groups during D0 to D30, D30 to D126, or overall, neither for proportions of patients transfused nor for numbers of units transfused per patient (supplemental Figure 1).
Proportions of transfused patients after starting rhEPO. (A) All conditionings together; (B) after MA conditioning (Cohort 1), after NMHCT with rhEPO started on D28 (Cohort 2), and after NMHCT when rhEPO initiated on D0 (Cohort 3).
Proportions of transfused patients after starting rhEPO. (A) All conditionings together; (B) after MA conditioning (Cohort 1), after NMHCT with rhEPO started on D28 (Cohort 2), and after NMHCT when rhEPO initiated on D0 (Cohort 3).
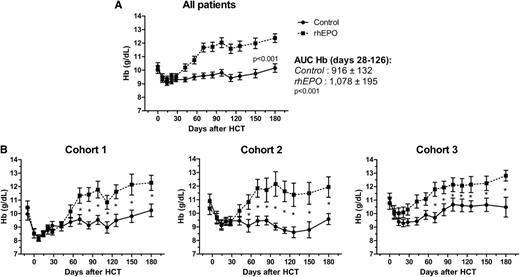
Figure 4 displays the evolution of Hb values with the Hb AUC. Mean Hb levels were higher in the EPO arm from week 2 after treatment initiation until D180. This was also true in the 3 cohorts separately.
Hb levels after transplantation. (A) Hb evolution of all transplants together (P < .001), (B) after MA regimen (Cohort 1), after NMHCT when rhEPO was started on D28 or D0, respectively (Cohorts 2 and 3). The control arm is indicated by a solid line, and the EPO arm by a dotted line.*P < .05.
Hb levels after transplantation. (A) Hb evolution of all transplants together (P < .001), (B) after MA regimen (Cohort 1), after NMHCT when rhEPO was started on D28 or D0, respectively (Cohorts 2 and 3). The control arm is indicated by a solid line, and the EPO arm by a dotted line.*P < .05.
After the reticulocyte surge on D21 (engraftment) in the 2 arms, we observed a second reticulocyte peak on D42 to D60 in EPO-treated patients (Figure 5A). Although the reticulocyte peak was transient, the sTfR increase was quite sustained (Figure 5B). Ferritin levels did not differ between the 2 arms, but Tsat decreased faster in the EPO arm (P < .05) (Figures 5C-D). The percentage of hypochromic RBCs (available only in cohort 3) was significantly higher in the EPO arm from D28 until D100 (Figure 5E).
Biological parameters after transplantation. Reticulocytes counts (A), sTfR values (B), ferritin levels (C), transferrin saturation (D), and percentage of hypochromic RBCs (E) after transplantation (all cohorts together except E [after NMHCT with rhEPO started on D0]). *P < .05.
Biological parameters after transplantation. Reticulocytes counts (A), sTfR values (B), ferritin levels (C), transferrin saturation (D), and percentage of hypochromic RBCs (E) after transplantation (all cohorts together except E [after NMHCT with rhEPO started on D0]). *P < .05.
Based on regression equations between Hct and log (Epo), predicted log (Epo) values were derived for each Hct and O/P ratios of observed/predicted Epo values were calculated.2 Epo O/P ratios before treatment were 0.83 ± 0.28 and 0.75 ± 0.21, respectively, in the 2 arms, in case of MA transplantation (NS); 0.83 ± 0.3 and 0.83 ± 0.28 on D28 after NMHCT (NS); and 1.05 ± 0.38 and 0.89 ± 0.27, respectively, on D0 of NMHCT in cohort 3 (NS).
All patients received QOL forms, but only a few filled them in (52 patients at baseline, 37 at D30 post-rhEPO, 25 at D70, and 15 at D150). It was therefore not possible to run meaningful comparisons between the 2 groups, and no significant relationship was found between Hb levels and QOL.
Safety issues
No reaction was reported after rhEPO administration. Only 2 patients, one in each arm, presented a thromboembolic event. The rates of arterial hypertension were 8% and 14% in the control and EPO arms, respectively (NS).
The incidence of infection was similar in the 2 arms. Thirty-two of 66 patients (48%) in the control arm and 37 of 63 patients (59%) in the EPO arm experienced at least one infectious episode between D28 and D126 after HCT (NS). The number of infections per patient between D28 and D126 (between D0 and D126 in cohort 3) was 0.7 ± 0.1 and 0.9 ± 0.1 in the control and EPO arms, respectively (NS). We observed more CMV reactivations in the EPO arm (48% of patients) than in the control arm (33%) (P = .0192). Reactivation rates were 0% vs 9% in CMV −/− pairs (NS) and 57% vs 47% in high-risk patients (NS) in the EPO and control arms, respectively.
Six patients in the control arm and 12 in the EPO arm relapsed on study (ie, until D126) (NS), whereas 13 and 7 died, respectively (NS). In multivariate analyses including the disease risk index, conditioning and treatment arm, only the disease risk index was associated with relapse. Seven patients experienced graft rejection: 4 in the control arm and 3 in the EPO arm (NS).
Functional iron deficiency was observed in 6 patients around D30 (N = 1), 60 (N = 1), or 100 (N = 4) posttransplant, all in the EPO arm (P = .0105). These patients received IV iron but most of them already had a brisk response to rhEPO therapy alone. One patient in the control arm presented absolute iron deficiency but did not receive any iron.
Finally, rhEPO therapy had no impact on platelet and WBC counts and differential, nor on C-reactive protein, creatinine value, or liver function tests.
Discussion
Despite the inadequacy of Epo response to anemia after MA allogeneic transplantation, most studies reported disappointing results when rhEPO was administered at very high doses from D1.10-12 However, we have shown in 2 pilot trials the potential efficacy of rhEPO initiated on D35 after MA HCT,13 or after NMHCT.15 The aim of this study was to confirm our previous observations in a prospective randomized trial, especially because there are no recommendations about rhEPO use after transplantation in published guidelines.20,21
Our study is the first randomized trial demonstrating that rhEPO is effective after allogeneic HCT, because 63% of patients receiving rhEPO vs 8% of controls achieved the primary end point (complete Hb correction) at a median of 90 days. All other analyses, including Hb response, Hb correction, and Hb AUC, were significantly in favor of rhEPO. This was true also in subanalysis of each cohort. This confirms the findings of our pilot trials after MA conditioning13 and after NMHCT.15 RhEPO was as efficient after MA and nonmyeloablative conditioning. Indeed, unlike in our previous study,5 Epo secretion, assessed by Epo O/P ratios, was low on D28 posttransplant, not only after MA but also after NMHCT. This could probably be explained by a higher proportion of NMHCT patients conditioned with only 2 Gy TBI in our previous report (50%).
Similar Hb responses have been reported in studies involving patients undergoing MA and NMHCT and treated with erythropoiesis-stimulating agents (ESA) for persisting anemia after transplantation.22,23 Another study after NMHCT also showed a positive impact of rhEPO therapy started on D1.24 Hb response was preceded by the expansion of erythropoietic activity, as assessed by sTfR levels, whereas reticulocyte counts were only transiently increased, as reported in previous trials after HCT.13-15,25 This parameter has indeed a poor quantitative value in assessing erythroid response.1,26
RBC transfusion requirements were also reduced, even though this was only borderline significant when the 3 cohorts were analyzed separately (mean reduction of ∼2.5 U per patient). Only 2 pilot studies published by us13 and another group23 reported similar reductions in transfusion requirements with rhEPO. Other randomized studies,10-12 except 2 much smaller ones,7,8 failed to demonstrate a benefit of high-dose IV rhEPO on transfusion requirements, but rhEPO was started early (on D0) and given only during 4 to 8 weeks when there are very few erythroid precursors in the bone marrow and a lot of endogenous Epo in the plasma. As aplasia is shortened, less profound or even sometimes absent, we asked whether initiating rhEPO on D0 could be effective after NMHCT. Although our pilot trial after NMHCT suggested decreased transfusion requirements only during the first month when rhEPO was started on D0,15 we could not confirm this in the current study. Pretransplant Hb levels were inversely correlated with transfusions requirements after NMHCT,27 but Hb levels before NMHCT did not differ between treatment arms and cohorts. Of course, the nonmyeloablative cohort starting rhEPO on D0 had higher pre-rhEPO values because their Hb had not yet decreased after conditioning. Moreover, transfusion rates were similar in the 2 rhEPO groups over the whole study period. Therefore, there was no benefit to start rhEPO on D0 rather than on D28.
Unlike other studies,24 ours did not provide systematic iron supplementation but monitored iron parameters to detect functional iron deficiency (defined by a Tsat <20%, with adequate ferritin values) that could impair response to rhEPO,1 and to provide IV iron in a targeted fashion. However, contrary to what is observed after autologous HCT,28 very few allogeneic transplant recipients developed low Tsat and required IV iron, and most already had a brisk Hb response with rhEPO alone. Therefore, hematopoietic response in this study was related to rhEPO therapy, with little contribution from IV iron. Because, based on Tsat, functional iron deficiency occurred rarely, the increased percentage of hypochromic RBC observed early in the EPO arm (Figure 5E) was probably not driven by iron deficiency but was related to the high percentage of reticulocytes (quite parallel in Figure 5A) in response to rhEPO.
Studies in cancer patients have demonstrated that Epo improved QOL mainly when Hb values reached 11 to 12 g/dL.29-31 Unfortunately, collected QOL data were insufficient to draw any conclusion. More stringent assessments of QOL should be pursued in further studies.
We did not encounter any toxicity of rhEPO therapy. Neutrophil and platelet engraftment were not influenced by rhEPO. Although meta-analyses have shown an increased risk of thromboembolic events with ESA in cancer patients,32 their rates were similar in the 2 arms in our trial. During the study, 14 patients (5 vs 9; NS) presented with arterial hypertension, requiring initiation of or an increase in antihypertensive medications. Many patients already had preexisting hypertension because of the effect of calcineurin inhibitors.33 The rates of infection, acute graft-vs-host disease, or graft rejection were also similar in the 2 arms. As predicted from its smaller proportion of patients at low risk for CMV reactivation (CMV-seronegative donor and recipient), there were more CMV reactivations in the EPO arm. However, reactivation rates were similar in both arms among low-risk and high-risk patients analyzed separately. Meta-analyses have also suggested shortened survival in cancer patients receiving ESA,34 but not in patients treated with concurrent chemotherapy.32,34 In our trial, short-term, disease-free, and overall survivals were comparable in the 2 arms (if anything, there were fewer deaths in the EPO arm; NS). Relapse rates were also similar in the 2 arms, with only the disease risk index being associated with relapse in multivariate analyses. However, long-term follow-up studies are needed to definitively rule out an effect on relapse and survival.
Our study has some limitations. The most important is the target Hb value of 13 g/dL. Indeed, current guidelines recommend a target Hb ∼12 g/dL20 or the lowest Hb level needed to avoid transfusions21 because of the possible association between high Hb levels and adverse outcomes. However, during our trial these restrictions were not yet effective. Thus, to rapidly achieve QOL improvement and transfusion independence, we aimed at Hb normalization. This is no longer our policy, even though we did not find any difference in adverse events or deaths in this trial. Conversely, when rhEPO was started on D28, only 5 patients had a Hb level higher than 11 g/dL (mean Hb ∼9 g/dL), in keeping with current guidelines for initiation of ESA therapy.20,21 In addition, a post hoc analysis limited to patients with a baseline Hb value <10 g/dL did not change the differences between the 2 arms. Nevertheless, even if we had adopted a much lower target Hb such as 9 g/dL, this would not have modified the impact of rhEPO on transfusions because their trigger was 8 g/dL. Second, although the overall patient population was quite large, patient numbers were limited in each cohort. Therefore, some could argue that there may be some undetected heterogeneity in our population for diagnosis, disease stage, conditioning regimen and intensity, donor type, and graft manipulation. However, this was also the case in all previous studies of rhEPO after HCT, and these pretransplant characteristics did not significantly differ between the 2 arms and did not affect posttransplant erythropoietic activity and response to rhEPO.13,15
Despite these limitations, our study is the first prospective randomized trial to demonstrate rhEPO efficacy in terms of Hb responses and limitation of transfusions in allogeneic transplantation after MA or NMHCT. This successful acceleration of erythroid recovery compared with previous studies relates to the initiation of EPO therapy at a more appropriate time frame after transplantation, ie, when endogenous Epo deficiency has become evident and not at an earlier time point when there are few erythroblasts to respond and very high serum Epo levels. However, there was no benefit in initiating treatment earlier, even in the case of NMHCT, thereby confirming in the setting of NMHCT the previous results after MA transplantation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
F.B. is senior research associate of the Fonds de la Recherche Scientifique and A.J. is a Fonds de la Recherche Scientifique-Televie student. We are grateful to Yvette Fairon and Olivier Dengis for their excellent technical assistance. Neorecormon was kindly provided by Roche Belgium.
Authorship
Contribution: Y.B. and F.B. designed the study; E.W., K.H., C.B., G.V., and A.J. collected the data; L.S., Y.B., and A.J. analyzed and interpreted the data; Y.B. and A.J. wrote the manuscript; and all authors approved the manuscript.
Conflict-of-interest disclosure: Y.B. is a consultant for and received honoraria and research funding from Amgen and Vifor. The remaning authors declare no competing financial interests.
Correspondence: Yves Beguin, University of Liège, Department of Hematology, Centre Hospitalier Universitaire Sart Tilman, 4000 Liège, Belgium; e-mail: yves.beguin@chu.ulg.ac.be.

![Figure 5. Biological parameters after transplantation. Reticulocytes counts (A), sTfR values (B), ferritin levels (C), transferrin saturation (D), and percentage of hypochromic RBCs (E) after transplantation (all cohorts together except E [after NMHCT with rhEPO started on D0]). *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/1/10.1182_blood-2014-01-546333/4/m_33f5.jpeg?Expires=1769370407&Signature=yasrwvzIu~6lLN7MK27XN-qgYw21qK-h9fJTjA-bUlG0wkuKmdv~me6fUCgSpZ5LC27sELsdZul-vN5-9Xtjmsc8QYAjfct0Jt1sIdu0Kdl~Fs059ESrpM4jooCZ7~M59oz1jkC2aCBuClG3wkZSJs~MjmQntc5~0uvshIY7Jnai1cAcTG-vhKKt5c3wsggvet~VwpbpjltwOnblrjstGroW~kh1FBcDO3z0~oHg0lHDlsZMLCdJime5PSLxM7Ygy3cSQzB3guTLNa-XKTAaG8dQ6ZiC7ro6zgwzJoi57dt84zQR1~9-E47UXRELLjN7N3Ji43wqTFNTztsDfa3srg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
