Key Points
O2− modifies B56δ at Y289 to block the PP2A holoenzyme assembly. This results in S70 Bcl-2 phosphorylation and promotes tumor chemoresistance.
Primary lymphomas with low SOD1 have high B56δ tyrosine nitration and S70pBcl-2.
Abstract
Bcl-2 is frequently overexpressed in hematopoietic malignancies, and selective phosphorylation at ser70 enhances its antiapoptotic activity. Phospho-ser70 is dephosphorylated by specific heterotrimers of protein phosphatase 2A (PP2A). We report here that a mild pro-oxidant intracellular milieu induced by either pharmacological inhibition or genetic knockdown of superoxide dismutase 1 (SOD1) inhibits the functional holoenzyme assembly of PP2A and prevents Bcl-2 ser70 dephosphorylation. This redox-dependent regulation of Bcl-2 phosphorylation is due to nitrosative modification of B56δ, which we identify as the regulatory subunit mediating PP2A-dependent Bcl-2 dephosphorylation. Redox inhibition of PP2A results from peroxynitrite-mediated nitration of a conserved tyrosine residue within B56δ (B56δY289). Although nitrated B56δY289 binds efficiently to ser70-phosphorylated Bcl-2, this specific modification inhibits the recruitment of the PP2A catalytic core (A and C subunits). Furthermore, inhibition of B56δY289 nitration restores PP2A holoenzyme assembly, thereby permitting S70 dephosphorylation of Bcl-2 and inhibiting its antiapoptotic activity. More important, in primary cells derived from clinical lymphomas, Bcl-2 phosphorylation at S70 directly correlates with B56δ nitration and repression of SOD1, but inversely correlates with B56δ interaction with the PP2A-C catalytic subunit. These data underscore the role of a pro-oxidant milieu in chemoresistance of hematopoietic and other cancers via selective targeting of tumor suppressors such as PP2A.
Introduction
PP2A is an essential phosphatase involved in numerous cellular processes, such as the regulation of cell-cycle progression, cytoskeleton dynamics, and cellular motility, and suppression of tumorigenesis via regulating cell proliferation and death pathways.1,2 To date, several important signaling pathways have been implicated in the anti-tumorigenic activity of PP2A. For instance, PP2A holoenzyme containing B56α was reported to be a direct regulator of c-Myc and Bcl-2, with the dephosphorylation of the former oncoprotein at Ser62 required for its degradation,3 whereas dephosphorylation of the latter at Ser70 leads to the inactivation of its antiapoptotic activity.4 Of note, inhibition of PP2A by okadaic acid or by polyamine depletion results in the specific accumulation of S70 phosphorylated Bcl-2 (S70pBcl-2), which in turn inhibited drug-induced apoptosis.5,6
Bcl-2 is a key regulator of apoptosis in various lymphoid and other malignancies. Bcl-2 inhibits cell death by sequestering pro-apoptotic proteins Bax and Bak at the outer mitochondrial membrane (OMM) and preventing the release of pro-apoptotic factors such as cytochrome c, AIF, and SMAC/DIABLO.7 The antiapoptotic activity of Bcl-2 is regulated by posttranslational modifications. Three residues (T69, S70, and S87) within the flexible loop domain of Bcl-28-10 are phosphorylated mainly by the mitogen-activated protein (MAP) kinases such as c-Jun N-terminal kinase, extracellular signal-regulated kinase (ERK), and p38.11 Mono-site (S70) phosphorylation increases the antiapoptotic activity of Bcl-2 by enhancing dimerization with Bax.5,6 The 2 adjacent phosphorylation sites provide additional functional modulation of Bcl-2 in a context-dependent manner.12
In addition to its canonical antiapoptotic activity, we have recently shown that Bcl-2 overexpression in hematopoietic tumors is associated with a pro-oxidant intracellular milieu, in particular an increased in intracellular superoxide (O2−) level. More importantly, inhibiting O2− production abrogated the antiapoptotic activity of Bcl-2.13 Although O2− is implicated in Bcl-2-mediated chemoresistance, the underlying molecular mechanism(s) is/are poorly understood.
Reactive oxygen species (ROS) such as O2− and H2O2 are important signaling molecules that have been implicated in various cellular processes. Redox homeostasis is maintained by antioxidant machineries such as superoxide dismutase (SOD), peroxiredoxins, glutathiones, and catalases. Defects in antioxidant defenses are invariably associated with pathological disease states; for example, SOD1 deficiency is linked with cancer and Alzheimer disease.14,15
Interestingly, ROS can regulate the activity of diverse kinases and phosphatases via diverse mechanisms.16-18 Here we show that physiologic increases in intracellular O2− induced by pharmacological inhibition and knockdown of SOD1 stabilize the antiapoptotic activity of Bcl-2 by inhibiting its dephosphorylation at S70. We also report that this is a function of selective nitration of a specific tyrosine residue (Y289) on the Bcl-2-bound B56δ subunit of PP2A; site-specific nitration of B56δ releases the PP2A catalytic subunit from Bcl-2, leading to increased S70 phosphorylation. Importantly, the nitrosative regulation of PP2A-mediated dephosphorylation of Bcl-2 is also observed in primary cells derived from clinical human lymphomas. These data provide mechanistic insights into the role of a pro-oxidant state in promoting chemoresistance via inactivating PP2A, thereby undermining its tumor suppressor activity.
Methods
Cell lines and cell culture
Jurkat, Hela, MDA-MB-231, and HK-1 tumor cell-lines were purchased from ATCC (Rockville, MD). All cell lines were cultured and maintained according to supplier’s recommendations.
Coimmunoprecipitation assay
Cells were lysed with coimmunoprecipitation (co-IP) lysis buffer and precleared with protein A agarose beads (Santa Cruz) under constant mixing at 4°C for an hour. Samples were then normalized, added with 3 μg of primary antibody, rotated overnight at 4°C, and then immunoprecipitated with protein A agarose beads. Subsequently, beads were washed three times and then analyzed via western blot.
Immunofluorescence confocal microscopy
Cells were fixed on slides and incubated with the primary antibodies of interests at the desired dilutions, followed by incubation with 1:200 dilution of Alexafluor-488 anti-mouse secondary antibody and Alexafluor-568 anti-rabbit secondary antibody (Invitrogen). Images were captured with Olympus Fluoview-FV1000 confocal microscope.
MTT cell viability assay
Cells were mixed with 4 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-dimethyltetrazolium bromide (MTT; Sigma Aldrich) in a 96-well format and incubated for 2 hours at 37°C. The formazan crystals formed were then spun down, dissolved in dimethylsulfoxide, and quantitated spectrophotometrically at an absorbance wavelength of 570 nm.
Crystal violet cell viability assay
Adherent cells were incubated with crystal violet solution for 10 minutes at room temperature. Nonviable cells were then washed off using distilled water and images were captured thereafter.
Construction of Y289F-B56δ mutant via site-directed mutagenesis
Site-directed mutagenesis of tyrosine-289 in B56δ to a phenylalanine residue was performed using the Quikchange II XL Site-directed Mutagenesis Kit provided by Stratagene.
Statistical analysis
All experiments were performed for at least 3 times, except for experiments involving clinical lymphoma biopsies. Numerical data were presented as mean ± standard deviation. The paired Student t test (2-tail, unequal variance) was used when comparisons were made between 2 groups. Statistical significance was set at P < .05. Refer to the detailed experimental protocols in the supplemental Experimental Procedures on the Blood Web site.
Results
S70 Bcl-2 phosphorylation is involved in O2−-mediated chemoresistance
To evaluate the effect of O2− on the phosphorylation of Bcl-2, intracellular O2− was induced in Jurkat cells by inhibition of SOD1 with diethyldithiocarbamate (DDC; 50-600 μM). Increase in O2− (supplemental Figure 1A) augmented S70pBcl-2 in a dose-dependent manner (Figure 1A), whereas the other 2 phosphorylation sites (T69 and S87) remained unchanged. A similar increase in S70pBcl-2 was also observed upon small interfering RNA (siRNA)-mediated silencing of SOD1 using 4 independent siRNA sequences (Figure 1B). Conversely, transient overexpression of SOD1 (Figure 1C) as well as the exogenous addition of bovine SOD1 (Figure 1D), significantly reduced the level of S70pBcl-2. Similarly, scavenging intracellular O2− (tiron; 1-5 mM) abrogated DDC-induced phosphorylation of S70 (Figures 1E; supplemental Figure 1B). Of note, O2−-induced phosphorylation of Bcl-2 at S70 is not exclusive to Jurkat cells, as evidenced in Hela cervical, MDA231 breast, and HK-1 nasopharyngeal cancer cell lines (supplemental Figure 1C-E).
O2− promotes chemoresistance through the induction of S70 Bcl-2 phosphorylation. (A) Bcl-2 phosphorylation status was examined in Jurkat cells treated with increasing doses of DDC for 4 hours. A total of 400 μM was selected as the working dose for subsequent experiments. (B) Western blot of Jurkat cells after genetic silencing of SOD1 using 4 independent siRNA sequences (denoted as siSOD1-A, B, C, and D). (C) S70pBcl-2 level was assessed in Jurkat cells transfected with vector (EV) or SOD1 plasmids. (D) Immunoblot of Jurkat cells treated with bovine SOD1 (bSOD1). bSOD1 appeared as faster migrating band under human SOD1. (E) Jurkat cells were pretreated for 1 hour with tiron, followed by 4 hours with DDC, and then lysates were immunoblotted for S70pBcl-2. (F) MTT cell viability assay of Jurkat cells pretreated with DDC (1 hour) followed by treatment with the indicated doses of chemotherapeutic agents (5FU, cisplatin, etoposide [Eto], and doxorubicin [Doxo]) for 18 hours. (G) MTT assay of Eto- or Doxo-treated Jurkat cells after knockdown of SOD1 using siSOD1A, B, or C. (H) MTT assay of 5FU- or cisplatin-treated Jurkat cells after pcDNA-SOD1 transfection or after the inhibition of reduced NADP oxidase-mediated O2− production via DPI (2.5 μM) pretreatment. (I) MTT assay of Jurkat cells expressing wild-type Bcl-2, S70A Bcl-2, or S70E Bcl-2 mutant after 18 hours of Eto treatment.
O2− promotes chemoresistance through the induction of S70 Bcl-2 phosphorylation. (A) Bcl-2 phosphorylation status was examined in Jurkat cells treated with increasing doses of DDC for 4 hours. A total of 400 μM was selected as the working dose for subsequent experiments. (B) Western blot of Jurkat cells after genetic silencing of SOD1 using 4 independent siRNA sequences (denoted as siSOD1-A, B, C, and D). (C) S70pBcl-2 level was assessed in Jurkat cells transfected with vector (EV) or SOD1 plasmids. (D) Immunoblot of Jurkat cells treated with bovine SOD1 (bSOD1). bSOD1 appeared as faster migrating band under human SOD1. (E) Jurkat cells were pretreated for 1 hour with tiron, followed by 4 hours with DDC, and then lysates were immunoblotted for S70pBcl-2. (F) MTT cell viability assay of Jurkat cells pretreated with DDC (1 hour) followed by treatment with the indicated doses of chemotherapeutic agents (5FU, cisplatin, etoposide [Eto], and doxorubicin [Doxo]) for 18 hours. (G) MTT assay of Eto- or Doxo-treated Jurkat cells after knockdown of SOD1 using siSOD1A, B, or C. (H) MTT assay of 5FU- or cisplatin-treated Jurkat cells after pcDNA-SOD1 transfection or after the inhibition of reduced NADP oxidase-mediated O2− production via DPI (2.5 μM) pretreatment. (I) MTT assay of Jurkat cells expressing wild-type Bcl-2, S70A Bcl-2, or S70E Bcl-2 mutant after 18 hours of Eto treatment.
Notably, as previously reported, an increase in intracellular O2− (DDC or siSOD1) significantly inhibited the antitumor activity of 2 commonly used chemotherapeutic agents, etoposide and doxorubicin (Figure 1F; supplemental Figure 1F-G). Conversely, overexpression of SOD1 or inhibition of O2− production via diphenyliodonium (DPI) pretreatment sensitized Jurkat cells to 5-fluorouracil (5FU) and cisplatin (Figure 1H); Jurkat cells are relatively refractory to 5FU and cisplatin (Figure 1F). So far, these data implicate S70 Bcl-2 phosphorylation in O2−-induced drug resistance.19-21
To directly test if S70 phosphorylation of Bcl-2 is required for O2−-mediated chemoresistance, we assessed the effect of transient expression of a phosphomimetic (S70E) or nonphosphorylatable (S70A) mutant of Bcl-2 on Jurkat cells sensitivity to etoposide. Whereas cells expressing phosphomimetic S70E Bcl-2 were significantly resistant to etoposide-induced cell death even in the absence of enhanced O2− production (Figure 1I), the expression of S70A Bcl-2 had the exact opposite effect and were no longer protected by enhanced O2−. These data indicate that S70pBcl-2 is a crucial mediator of O2−-induced chemoresistance in tumor cells.
O2− inhibits the recruitment of PP2A to mitochondrial Bcl-2
Regulation of protein phosphorylation is achieved by a dynamic balance between the activities of specific kinases and phosphatases. Although the stress-activated MAP kinases are frequently implicated in the regulation of Bcl-2 phosphorylation,11 neither c-Jun N-terminal kinase nor p38 were activated by increased O2− (supplemental Figure 2A). Although ERK1/2 was activated upon O2− induction in Jurkat cells, inhibition of ERK1/2 with PD98059 had no effect on S70 phosphorylation. These data suggest against the involvement of MAP kinases in O2−-induced S70 Bcl2 phosphorylation (supplemental Figure 2B).
To evaluate if O2−-induced upregulation of S70pBcl-2 could be due to an inactivation of Bcl-2 phosphatases, we tested if DDC treatment could affect the interaction of Bcl-2 with the catalytic subunits of 2 major Bcl-2 phosphatases, PP2A (PP2A-C) and PP1 (PP1-C). Although Bcl-2 co-immunoprecipitated with both PP2A-C and PP1-C (Figure 2A), only the interaction of Bcl-2 with PP2A-C was disrupted upon O2− induction. This was accompanied by a concomitant increase in S70pBcl-2 (Figure 2A). Similar findings were observed in Hela and MDA231 cell lines (Figure 2B) or when endogenous PP2A-C was immunoprecipitated (Figure 2C). Further supporting the hypothesis that Bcl-2 is downstream of PP2A, we found that pharmacological inhibition (okadaic acid) or activation (FTY720) of PP2A respectively enhanced or decreased the phosphorylation of Bcl-2 at S70 (supplemental Figure 2C). These data suggest that O2−-induced upregulation of S70pBcl-2 could be due to the inhibition of PP2A-C mediated dephosphorylation of Bcl-2.
O2− inhibits PP2A-mediated dephosphorylation of Bcl-2 at S70. (A-C) Lysates from DDC-treated Jurkat, Hela, and MDA231 were immunoprecipitated (IP) and immunoblotted (IB) using the indicated antibodies. Input refers to whole cell lysates that are not subjected to IP procedures. Immunoglobulin G (IgG) refers to untreated lysates that were incubated with nonspecific IgG primary antibodies. (D) Mitochondria isolated from MDA231 or Jurkat cells were incubated with proteinase K for 25 minutes followed by lysis and IB for the indicated proteins. As a control, incubation of mitochondria with Triton-X100 resulted in the digestion of SOD2 (matrix protein) and COX Va (inner mitochondrial membrane [IMM] protein). (E) Mitochondrial/cytosolic fractions from DDC-treated Jurkat or MDA231 cells (with or without tiron pretreatment) were IB for the indicated proteins. SOD1 was employed as a cytosolic marker, whereas prohibitin, an IMM protein, served as a mitochondrial marker. (F) Confocal fluorescence imaging studies of DDC-treated MDA231 cells. Colocalization of Bcl-2 (red) and PP2A-C (green) appears as yellow punctate (red arrows). (G) Confocal imaging of Jurkat cells 48 hours after silencing of SOD1. Selected cells were enlarged for greater clarity.
O2− inhibits PP2A-mediated dephosphorylation of Bcl-2 at S70. (A-C) Lysates from DDC-treated Jurkat, Hela, and MDA231 were immunoprecipitated (IP) and immunoblotted (IB) using the indicated antibodies. Input refers to whole cell lysates that are not subjected to IP procedures. Immunoglobulin G (IgG) refers to untreated lysates that were incubated with nonspecific IgG primary antibodies. (D) Mitochondria isolated from MDA231 or Jurkat cells were incubated with proteinase K for 25 minutes followed by lysis and IB for the indicated proteins. As a control, incubation of mitochondria with Triton-X100 resulted in the digestion of SOD2 (matrix protein) and COX Va (inner mitochondrial membrane [IMM] protein). (E) Mitochondrial/cytosolic fractions from DDC-treated Jurkat or MDA231 cells (with or without tiron pretreatment) were IB for the indicated proteins. SOD1 was employed as a cytosolic marker, whereas prohibitin, an IMM protein, served as a mitochondrial marker. (F) Confocal fluorescence imaging studies of DDC-treated MDA231 cells. Colocalization of Bcl-2 (red) and PP2A-C (green) appears as yellow punctate (red arrows). (G) Confocal imaging of Jurkat cells 48 hours after silencing of SOD1. Selected cells were enlarged for greater clarity.
PP2A is reported to bind to Bcl-2 at the OMM.6 We confirmed that the A and C core subunits of PP2A localize to the OMM of purified mitochondria together with Bcl-2 (Figure 2D). Confocal microscopy using Mitotracker also demonstrated that majority of Bcl-2 is localized at the mitochondria (supplemental Figure 2D). We next tested if the mitochondrial localization of the AC core enzyme was affected by increased intracellular O2−. Interestingly, in both Jurkat and MDA231 cells, mitochondrial PP2A-A and PP2A-C expression decreased following an increase in intracellular O2−, concomitant with phosphorylation of Bcl-2 S70, and both effects were reversed by the O2− scavenger tiron (Figure 2E). These data were further corroborated by confocal microscopy, demonstrating that DDC treatment or SOD1 knockdown decreased the colocalization of PP2A-C with Bcl-2 (Figure 2F-G).
O2− inhibits the holoenzyme assembly of PP2A
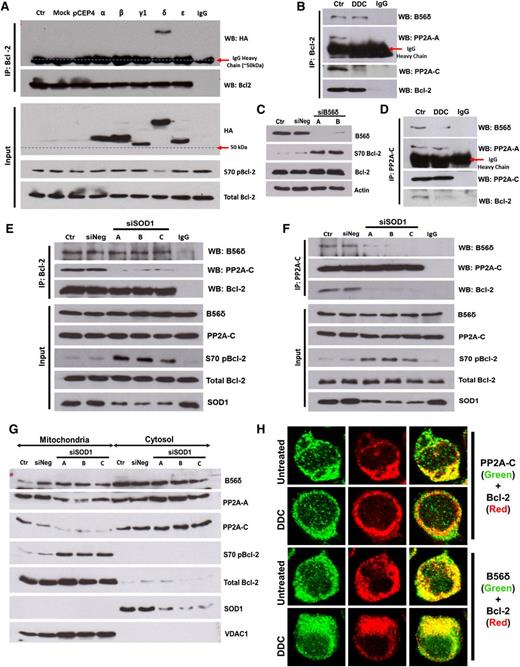
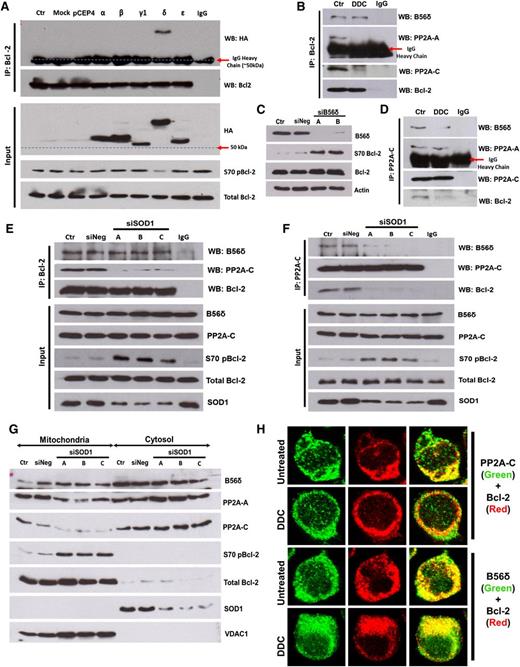
PP2A is a heterotrimer, with a core A and C heterodimer whose substrate specificity is determined by its B regulatory subunit.22 B56α has been previously identified as a regulatory subunit responsible for PP2A-mediated dephosphorylation of Bcl-2.4 Because the B56 family of targeting subunits can be redundant and tissue-specific,23-25 we tested the interaction of all PP2A-B56 subunits with Bcl-2 in our system. Co-IP assays revealed HA-B56δ as a major interacting partner of Bcl-2 in both Jurkat (Figure 3A) and MDA231 cells (supplemental Figure 3A). This interaction appears physiologic, because immunoprecipitation of endogenous Bcl-2 also pulled down endogenous B56δ (Figure 3B; supplemental Figure 3B). Consistent with the model that B56δ recruits PP2A to dephosphorylate Bcl-2, expression of HA-B56δ resulted in a decline in S70pBcl-2 level (Figure 3A), whereas silencing of B56δ resulted in increased S70pBcl-2 (Figure 3C). These data indicate that a B56δ-containing PP2A heterotrimer (PP2AB56δ) is involved in the dephosphorylation of Bcl-2 at S70 in our model of study.
O2− augmented the level of S70pBcl-2 by inhibiting the holoenzyme assembly of PP2AB56δ. (A) Jurkat cells expressing hemagglutinin (HA)-tagged B56 family members (α, β, γ1, δ, and ε isoforms) were subjected to co-IP analysis using Bcl-2 as bait and IB for the indicated proteins. (B) Lysates from DDC-treated Jurkat cells were IP and IB with the indicated antibody (Ab). (C) Jurkat cells transfected with B56δ-specific siRNA (sequence A or B) were lysed and IB for the indicated proteins. (D-F) Lysates from DDC-treated or SOD1-silenced Jurkat cells were IP and IB using the indicated Abs. (G) Mitochondrial/cytosolic fractions from SOD1-silenced Jurkat cells were IB for the indicated proteins. The OMM protein VDAC1 served as a mitochondrial marker. (H) Confocal indirect immunofluorescence imaging of DDC-treated Jurkat cells. PP2A-C (left column) or B56δ (right column) is stained in green; Bcl-2 is stained in red. Colocalization of Bcl-2 and PP2A-C (left column) or B56δ (right column) appears yellow.
O2− augmented the level of S70pBcl-2 by inhibiting the holoenzyme assembly of PP2AB56δ. (A) Jurkat cells expressing hemagglutinin (HA)-tagged B56 family members (α, β, γ1, δ, and ε isoforms) were subjected to co-IP analysis using Bcl-2 as bait and IB for the indicated proteins. (B) Lysates from DDC-treated Jurkat cells were IP and IB with the indicated antibody (Ab). (C) Jurkat cells transfected with B56δ-specific siRNA (sequence A or B) were lysed and IB for the indicated proteins. (D-F) Lysates from DDC-treated or SOD1-silenced Jurkat cells were IP and IB using the indicated Abs. (G) Mitochondrial/cytosolic fractions from SOD1-silenced Jurkat cells were IB for the indicated proteins. The OMM protein VDAC1 served as a mitochondrial marker. (H) Confocal indirect immunofluorescence imaging of DDC-treated Jurkat cells. PP2A-C (left column) or B56δ (right column) is stained in green; Bcl-2 is stained in red. Colocalization of Bcl-2 and PP2A-C (left column) or B56δ (right column) appears yellow.
We next tested if increase in O2− affected the binding of B56δ to Bcl-2. Increasing intracellular O2− decreased the interaction of Bcl-2 with PP2A-A and PP2A-C subunits (Figure 3B,D), but not B56δ (Figure 3B,E). These data indicate that O2− inhibits the association of Bcl-2-bound B56δ from PP2A-AC heterodimer. Consistent with this, PP2A-C binding to both Bcl-2 and B56δ was reduced under similar conditions (Figure 3D,F), whereas the PP2A-AC heterodimer remained intact despite not being in association with B56δ (Figure 3D). In purified mitochondria, B56δ abundance was unaffected by siSOD1 despite a marked decrease in the amount of mitochondrial PP2A-A and -C (Figure 3G). Likewise, DDC treatment reduced the colocalization of Bcl-2 with PP2A-C but not with B56δ (Figure 3H). Taken together, the data suggest that increased intracellular O2− inhibits the recruitment of PP2A-AC heterodimer to Bcl-2-bound B56δ.
ONOO− is downstream of O2− and modifies B56δ to block PP2A holoenzyme assembly
We sought to understand how increased O2− production might inhibit the assembly of the PP2A heterotrimer. O2− does not readily react with most biological molecules,26 thus strongly suggesting the involvement of downstream products of O2− such as peroxynitrite (ONOO−) and H2O2. Because O2− production was induced by preventing the dismutation of O2− to H2O2, the involvement of H2O2 is unlikely. The alternative reaction of O2− with nitric oxide (NO) to form ONOO− may therefore be crucial for O2−-mediated signaling on PP2A.
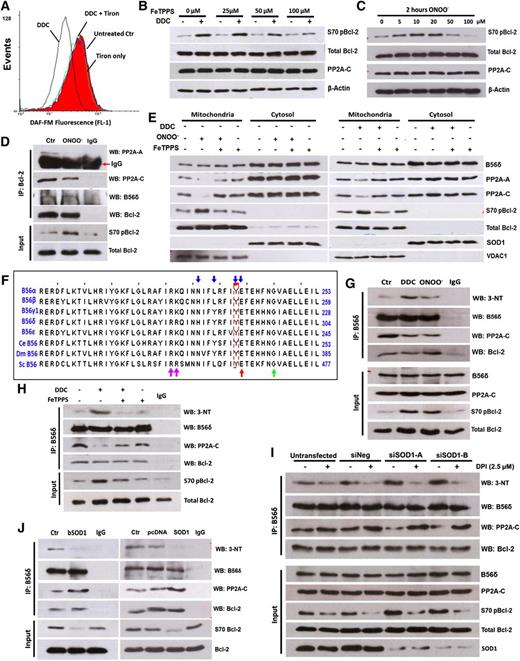
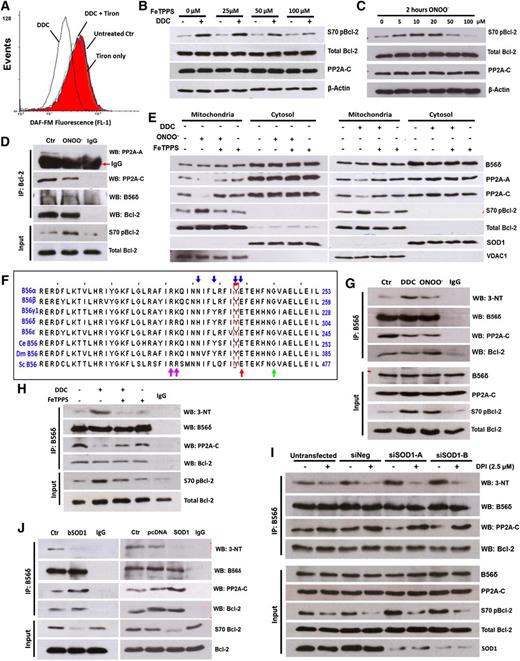
The production of ONOO− resulting from the reaction of O2− with NO consumes intracellular NO. Because there is no reliable commercial ONOO− probe, we sought to measure the decrease of intracellular NO as a surrogate indicator of ONOO− production upon O2− induction. Indeed, a decline in the fluorescence of the NO-sensitive probe, DAF-FM, was clearly detected upon an increase in O2− in tumor cells (Figure 4A; supplemental Figure 4A). Pretreatment with the O2− scavenger tiron abrogated the drop in NO induced by DDC in both cell lines, consistent with the generation of ONOO−.
ONOO− is downstream of O2− in the inhibition of PP2AB56δ holoenzyme assembly. (A) Intracellular NO was measured in DDC-treated Jurkat cells using a NO-sensitive probe, DAF-FM. (B) Jurkat cells were pretreated with FeTPPS for 1 hour, followed by DDC treatment, and then IB for the indicated proteins. (C) S70pBcl-2 status was assessed in Jurkat cells after a 2-hour treatment with the indicated concentrations of ONOO−. (D) Jurkat cells were treated with 10 μM ONOO− for 2 hours and harvested for co-IP assay with Bcl-2 as bait. (E) IB analysis of mitochondrial fractions from Jurkat cells preincubated with 100 μM FeTPPS followed by DDC or ONOO− treatment. (F) Multiple sequence alignment of B56 regulatory subunits of both mammalian and nonmammalian origin. The nitration-prone tyrosine residue (Y) identified in B56 family members is boxed in red. Blue arrows denote residues that are implicated in the interaction of B56 subunits with PP2A-A.28 Adjacent residues proposed to predispose a tyrosine residue toward nitrative modification process are indicated by red, green, and purple arrows (refer to supplemental Figure 5C for details). Multisequence alignment analysis was performed via Jalview 2.7. (G-H) Lysates from DDC (with or without FeTPPS pretreatment) and ONOO−-treated Jurkat cells were IP using anti-B56δ Ab and IB for 3-NT and other indicated proteins. (I) Twenty-four hours after SOD1 silencing, Jurkat cells were treated with 2.5 μM DPI for an additional 24 hours and harvested for co-IP analysis using the indicated Abs. (J) Lysates from bSOD1 treated (16 hours, 1 kU) or SOD1-overexpressing Jurkat cells were IP using anti-B56δ Ab and IB for 3-NT and other indicated proteins.
ONOO− is downstream of O2− in the inhibition of PP2AB56δ holoenzyme assembly. (A) Intracellular NO was measured in DDC-treated Jurkat cells using a NO-sensitive probe, DAF-FM. (B) Jurkat cells were pretreated with FeTPPS for 1 hour, followed by DDC treatment, and then IB for the indicated proteins. (C) S70pBcl-2 status was assessed in Jurkat cells after a 2-hour treatment with the indicated concentrations of ONOO−. (D) Jurkat cells were treated with 10 μM ONOO− for 2 hours and harvested for co-IP assay with Bcl-2 as bait. (E) IB analysis of mitochondrial fractions from Jurkat cells preincubated with 100 μM FeTPPS followed by DDC or ONOO− treatment. (F) Multiple sequence alignment of B56 regulatory subunits of both mammalian and nonmammalian origin. The nitration-prone tyrosine residue (Y) identified in B56 family members is boxed in red. Blue arrows denote residues that are implicated in the interaction of B56 subunits with PP2A-A.28 Adjacent residues proposed to predispose a tyrosine residue toward nitrative modification process are indicated by red, green, and purple arrows (refer to supplemental Figure 5C for details). Multisequence alignment analysis was performed via Jalview 2.7. (G-H) Lysates from DDC (with or without FeTPPS pretreatment) and ONOO−-treated Jurkat cells were IP using anti-B56δ Ab and IB for 3-NT and other indicated proteins. (I) Twenty-four hours after SOD1 silencing, Jurkat cells were treated with 2.5 μM DPI for an additional 24 hours and harvested for co-IP analysis using the indicated Abs. (J) Lysates from bSOD1 treated (16 hours, 1 kU) or SOD1-overexpressing Jurkat cells were IP using anti-B56δ Ab and IB for 3-NT and other indicated proteins.
We next investigated if ONOO− was responsible for O2−-mediated induction of S70pBcl-2 in tumor cells. O2−-mediated phosphorylation of Bcl-2 at S70 was blocked by FeTPPS (a porphyrin-based catalyst of ONOO− degradation) pretreatment in a dose-dependent manner (Figure 4B). Removal of ONOO− by FeTPPS also sensitized Jurkat cells to 5FU and cisplatin (supplemental Figure 4E). Conversely, addition of exogenous ONOO− caused a dose-dependent increase in S70pBcl-2 that peaked at 10 μM (Figure 4C). This was accompanied by a decline in the interaction between Bcl-2 and the PP2A-AC heterodimer (Figure 4D; supplemental Figure 4B), a drop in mitochondria localization of PP2A-A and -C (Figure 4E), and an augmented resistance against drug-induced apoptosis (supplemental Figure 4C-D). These data directly implicate ONOO− as an essential intermediate in the O2−-mediated induction of S70pBcl-2 in tumor cells.
We next sought to identify the precise biochemical modification by ONOO− that resulted in the decreased interaction of the PP2A-AC heterodimer with Bcl-2-bound B56δ. We considered it unlikely that the core AC heterodimer enzyme would be a target of O2−/ONOO− because inhibition of global PP2A enzymatic activity would be highly toxic. We therefore examined if B56δ could be a target for nitrosative modification. The 2 most common protein modifications by ONOO− are the S-nitrosylation of cysteine residues and the nitration of tyrosine residues.26 We examined a multiple sequence alignment of various B56 isoforms (both mammalian and nonmammalian origins) for highly conserved cysteine or tyrosine residues that could serve as candidate nitrosative modification sites. Interestingly, we identified a highly conserved tyrosine residue (corresponding to Y289 of human B56δ; Figure 4F) that not only falls within the A subunit binding domain of the B56 subunits,27 but also serves as 1 of the interacting residues between the B56 isoforms and the A-scaffolding subunit.28,29 This tyrosine residue was an attractive target for additional study because nitrative modification of this residue would be predicted to block the interaction between PP2A-AC heterodimers and B56 subunits bound to specific targets such as Bcl-2.
Serendipitously, analysis of sequence adjacent to Y289 revealed that this tyrosine residue is also particularly prone to nitrative processes. Protein tyrosine nitration is governed by the local context of the tyrosine, and specific criteria that identify favorable sites have been defined (supplemental Figure 5C).30,31 Notably, all of these criteria favor modification of Y289 in B56δ (Figure 4F; supplemental Figure 5A-B).
Based on these in silico findings, we tested if B56δ was tyrosine-nitrated in an ONOO−-dependent manner. Indeed, 3-nitrotyrosine (3-NT) was detected in B56δ immunoprecipitated from Jurkat and Hela lysates and was markedly augmented upon an increase in O2− or ONOO− treatment (Figure 4G; supplemental Figure 5D). Importantly, O2−/ONOO−-induced B56δ tyrosine nitration was always paralleled by a decline in B56δ-PP2A-C interaction and an increase in S70pBcl-2, whereas the interaction between B56δ and Bcl-2 remained constant despite the redox modification of B56δ (Figure 4G; supplemental Figure 5D). In line with this, FeTPPS pretreatment blocked the increase in DDC-induced B56δ tyrosine nitration, restored the binding of B56δ to PP2A-C, and allowed for the dephosphorylation of S70 of Bcl-2 (Figure 4H). Similar results were obtained when DPI was used to block the increase in intracellular O2− levels induced by SOD1 knockdown (Figure 4I; supplemental Figure 5E). Conversely, overexpression of SOD1 resulted in a decrease in B56δ tyrosine nitration accompanied by an increase in the formation of PP2A-AC-B56δ heterotrimers and dephosphorylation of S70pBcl-2 (Figure 4J). The effect of O2− on PP2AB56δ holoenzyme assembly is also reflected by the changes in its phosphatase activity. DDC treatment resulted in a decline in PP2AB56δ activity, whereas pretreatment with DPI or FeTPPS ameliorated the effect of DDC (supplemental Figure 5F). Collectively, these data indicate that O2−-induced S70 phosphorylation of Bcl-2 is driven by ONOO−-mediated tyrosine nitration of B56δ and the consequential impairment of PP2A holoenzyme assembly.
PP2A-B56δ is nitrated at Tyr289 by ONOO−
To further investigate if Y289 of B56δ (B56δY289) was indeed nitrated as predicted by our in silico analysis, we generated a custom antibody specifically raised against a synthetic peptide comprising nitrated B56δY289 and flanking sequence (supplemental Figure 6A). Affinity-purified antibody detected a single prominent band corresponding to the molecular mass of B56δ (69.9 kDa) (Figure 5A), indicating that B56δY289 was nitrated in Jurkat cells. More importantly, nitrated B56δY289 was markedly enhanced by increasing intracellular O2− or ONOO− and paralleled the abundance of S70pBcl-2 (Figure 5A). Moreover, degradation of ONOO− by FeTPPS abrogated O2−-induced B56δY289 nitration. However, inhibition of O2− production did not affect the ONOO−-induced nitration of B56δY289 nor the upregulation of S70pBcl-2, further supporting the model that ONOO- is downstream of O2− in the modification of B56δ and regulation of S70 Bcl-2 phosphorylation.
Inhibition of PP2AB56δ by O2− is mediated by the nitration of B56δ at Y289. (A) Nitration status of B56δY289 (denoted as 3NO2-Y289) was evaluated in Jurkat cells subjected to the indicated treatments using a custom-made Ab raised in rabbits immunized with peptides containing nitrated B56δY289. The detected protein band migrates at a similar rate as endogenous B56δ on sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Nitration of B56δ did not appear to alter the migration rate of B56δ because only a single band was detected by B56δ antibody. (B) Jurkat cells expressing WT-B56δ or Y289F-B56δ were treated with DDC for 4 or 8 hours and then lysates were IB for the indicated proteins. pCEP4, empty vector. (C) Jurkat cells expressing WT-B56δ or Y289F-B56δ were treated with 8 hours DDC, lysed, IP using anti-HA Ab, and IB for the indicated proteins. (D) WT-B56δ– or Y289F-B56δ–expressing Jurkat cells were pretreated for 1 hour with DDC and then treated with either 2.5 μM Eto or 1 μM Doxo for 18 hours. Cell viability was assessed via MTT assay thereafter. (E) Jurkat cells were transfected with first with SOD1-targeted siRNA (siA or siB), and 24 hours later, with plasmids expressing WT-B56δ or Y289F-B56δ. Twenty-four hours later, lysates were subjected to co-IP analysis as in panel C. (F) Jurkat cells were subjected to the same transfection procedures as in panel E, followed by 18 hours of Eto (2.5 μM) or Doxo (1 μM) treatment. Cell viability was then assessed via MTT assay.
Inhibition of PP2AB56δ by O2− is mediated by the nitration of B56δ at Y289. (A) Nitration status of B56δY289 (denoted as 3NO2-Y289) was evaluated in Jurkat cells subjected to the indicated treatments using a custom-made Ab raised in rabbits immunized with peptides containing nitrated B56δY289. The detected protein band migrates at a similar rate as endogenous B56δ on sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Nitration of B56δ did not appear to alter the migration rate of B56δ because only a single band was detected by B56δ antibody. (B) Jurkat cells expressing WT-B56δ or Y289F-B56δ were treated with DDC for 4 or 8 hours and then lysates were IB for the indicated proteins. pCEP4, empty vector. (C) Jurkat cells expressing WT-B56δ or Y289F-B56δ were treated with 8 hours DDC, lysed, IP using anti-HA Ab, and IB for the indicated proteins. (D) WT-B56δ– or Y289F-B56δ–expressing Jurkat cells were pretreated for 1 hour with DDC and then treated with either 2.5 μM Eto or 1 μM Doxo for 18 hours. Cell viability was assessed via MTT assay thereafter. (E) Jurkat cells were transfected with first with SOD1-targeted siRNA (siA or siB), and 24 hours later, with plasmids expressing WT-B56δ or Y289F-B56δ. Twenty-four hours later, lysates were subjected to co-IP analysis as in panel C. (F) Jurkat cells were subjected to the same transfection procedures as in panel E, followed by 18 hours of Eto (2.5 μM) or Doxo (1 μM) treatment. Cell viability was then assessed via MTT assay.
We next asked if nitration of Y289 is required for O2−-induced upregulation of S70pBcl-2. Wild-type (WT-B56δ) or Y289F mutant (Y289F-B56δ) HA-B56δ (supplemental Figure 6B) was expressed in Jurkat cells and the response to O2− assessed. Overexpression of WT-B56δ resulted in a modest delay in DDC-induced S70 Bcl-2 phosphorylation compared with controls, whereas expression of Y289F-B56δ completely abolished O2−-induced S70 phosphorylation (Figure 5B). In addition, we tested if Y289F mutation of B56δ could reverse the effects of O2− on PP2A. Expression of Y289F-B56δ blocked O2− or ONOO−-induced nitration of B56δ, and at the same time blocked phosphorylation of Bcl-2 at S70 (Figure 5C,E; supplemental Figure 6C). O2− reduced the interaction of B56δ with PP2A-C, and this was prevented by expression of Y289F-B56δ (Figure 5C, compare lane 6 with lane 8). Furthermore, the protective effect of O2− on drug-induced cell death is alleviated in cells expressing Y289F-B56δ (Figure 5D,F). These data confirm B56δY289 nitration as a critical step in O2−-mediated inhibition of both PP2AB56δ holoenzyme assembly and the Bcl-2 phosphatase activity that accompanies it.
Low SOD1 expression correlates with elevated B56δ tyrosine nitration and S70pBcl-2 in primary lymphoma biopsies
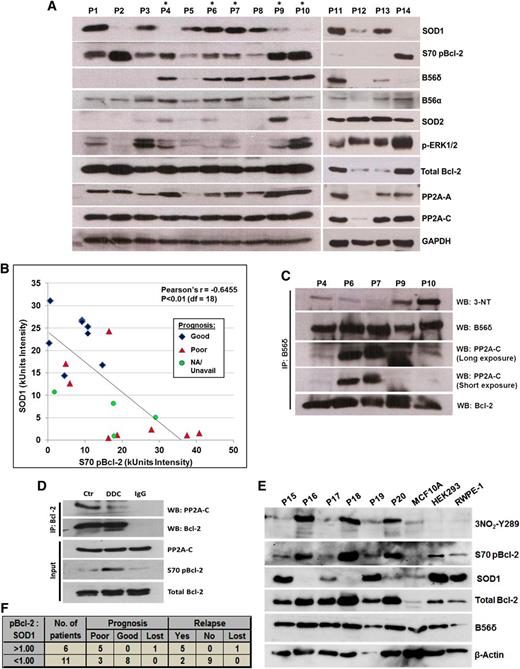
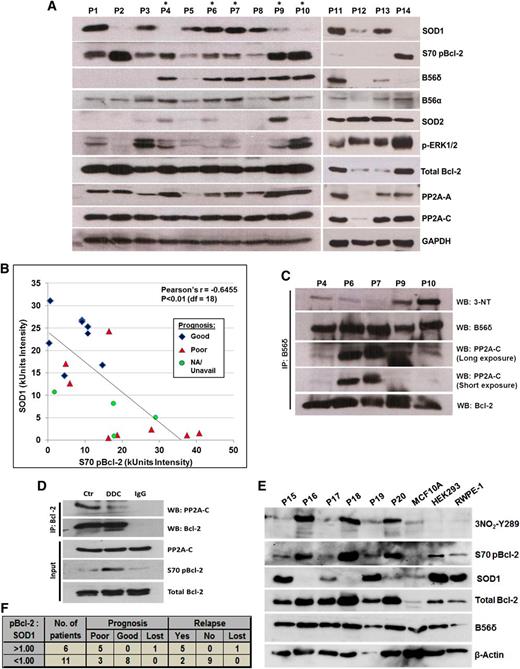
To assess the in vivo relevance of these findings, we analyzed the expression and phosphorylation status of relevant proteins in primary patient-derived lymphoma samples. Consistent with the in vitro findings, S70pBcl-2 levels were inversely related to SOD1 abundance (Figure 6A-B; supplemental Figure 7). There were, however, 2 outliers, P1 and P3, in which S70pBcl-2 was observed despite the high expression of SOD1. Notably, P1 and P3 lack B56δ expression, which may explain why S70 is not dephosphorylated.
In vivo evidence of an inverse relationship between SOD1 expression and the level of both S70pBcl-2 and B56δ tyrosine nitration. (A) Western blot (WB) analysis of clinical lymphoma biopsies that are arbitrarily numbered as P1-P14. More complete clinical diagnoses for these samples are described in supplemental Figure 7. Lymphoma biopsies denoted with an asterisk were derived from tumors sufficiently large for further analysis via co-IP assay. (B) Scatter plot analysis of densitometry intensity values of SOD1 and S70pBcl-2. Densitometry values of each sample can be found in supplemental Figure 7. Statistical significance was calculated using Pearson correlation coefficient with a degree of freedom of 18. (C) B56δ was IP from lysates denoted with asterisk and IB for the indicated proteins. (D) This experiment was performed using a rare, huge mantle cell lymphoma that could be sufficiently separated into 3 samples for co-IP analysis. Single cell suspension was prepared, treated for 4 hours with 400 μM DDC, and then harvested for co-IP assay using the indicated Abs. (E) WB analysis of 6 other clinical lymphoma biopsies denoted as P15-P20 with 3 other nonmalignant cell lines: MCF10A breast epithelial cells, HEK293 human embryonic kidney cells, and RWPE-1 prostate epithelial cells. (F) The ratio of S70pBcl-2:SOD1 was calculated using the densitometry value of each protein in supplemental Figure 7 and subsequently correlated with the prognosis or relapse status of each tumor. A ratio greater than 1 would mean a greater abundance of S70pBcl-2 relative to SOD1 and vice versa. Prognosis records, clinical data, and diagnosis of all primary samples can be found in supplemental Figure 7. Three samples—P9, P13, and P20—were diagnosed as reactive lymphoid hyperplasia (not lymphomas) and therefore excluded from the analysis. Clinical records for some patients were unavailable or lost because of a loss of follow-up or some biopsies may have been donated by overseas patients and could not be retrieved.
In vivo evidence of an inverse relationship between SOD1 expression and the level of both S70pBcl-2 and B56δ tyrosine nitration. (A) Western blot (WB) analysis of clinical lymphoma biopsies that are arbitrarily numbered as P1-P14. More complete clinical diagnoses for these samples are described in supplemental Figure 7. Lymphoma biopsies denoted with an asterisk were derived from tumors sufficiently large for further analysis via co-IP assay. (B) Scatter plot analysis of densitometry intensity values of SOD1 and S70pBcl-2. Densitometry values of each sample can be found in supplemental Figure 7. Statistical significance was calculated using Pearson correlation coefficient with a degree of freedom of 18. (C) B56δ was IP from lysates denoted with asterisk and IB for the indicated proteins. (D) This experiment was performed using a rare, huge mantle cell lymphoma that could be sufficiently separated into 3 samples for co-IP analysis. Single cell suspension was prepared, treated for 4 hours with 400 μM DDC, and then harvested for co-IP assay using the indicated Abs. (E) WB analysis of 6 other clinical lymphoma biopsies denoted as P15-P20 with 3 other nonmalignant cell lines: MCF10A breast epithelial cells, HEK293 human embryonic kidney cells, and RWPE-1 prostate epithelial cells. (F) The ratio of S70pBcl-2:SOD1 was calculated using the densitometry value of each protein in supplemental Figure 7 and subsequently correlated with the prognosis or relapse status of each tumor. A ratio greater than 1 would mean a greater abundance of S70pBcl-2 relative to SOD1 and vice versa. Prognosis records, clinical data, and diagnosis of all primary samples can be found in supplemental Figure 7. Three samples—P9, P13, and P20—were diagnosed as reactive lymphoid hyperplasia (not lymphomas) and therefore excluded from the analysis. Clinical records for some patients were unavailable or lost because of a loss of follow-up or some biopsies may have been donated by overseas patients and could not be retrieved.
Of the 14 primary lymphoma samples tested, 5 (denoted with *) were derived from tumors that were sufficiently large for further experimental analysis via co-IP assay. Using these lymphoma biopsies, we asked if B56δ was tyrosine nitrated in primary lymphoma tumors and if this correlated with S70pBcl-2. As predicted, a robust interaction between B56δ and Bcl-2 was detected in all 5 samples (Figure 6C). B56δ tyrosine nitration was also detected in tumor biopsies derived from P9 and P10, and to a lesser extent in tumors P6 and P7; this correlated with the level of SOD1. Importantly, the highly nitrated B56δ in P9 and P10 interacted poorly with PP2A-C (Figure 6C), which correlated with increased S70pBcl-2 in the immunoblots in Figure 6A. In 2 Hodgkin lymphoma samples (P6 and P7) with abundant SOD1, B56δ nitration was low, and B56δ interacted with the PP2A-C subunit. P4 was an exception. B56δ was unable to bind PP2A-C despite being poorly nitrated, suggesting a potential heterogeneity in the regulation of PP2A subunits in primary tumors. In P4, it is plausible that B56δ may have lost its initial ability to bind PP2A-C, rendering B56δ tyrosine nitration less critical in the regulation of S70pBcl-2. Whether this phenomenon results from an inherent mutation on B56δ or PP2A-C warrants further investigation. We also tested if the suppression of SOD1 activity elicited a similar signaling response in primary cells from a mantle lymphoma. As predicted, PP2A-C coprecipitated with Bcl-2 and treatment with DDC blocked the interaction and augmented the S70pBcl-2 level (Figure 6D).
To check if B56δY289 nitration is also synchronized with the inverse status of S70pBcl-2/SOD1, we analyzed the levels of nitrated B56δY289, S70pBcl-2, and SOD1 in 6 additional (P15-P20) primary lymphoma samples. Consistent with our in vitro findings, high levels of nitrated B56δY289 correlated well with elevated S70pBcl-2 and low SOD1 (P16, P18, and P20), whereas the converse is true for samples with low nitrated B56δY289 level (P15, P17, and P19) (Figure 6E). Of note, nitrated B56δY289 could not be detected in 3 other nonmalignant cell lines, whereas little or no S70pBcl-2 was present in these cell lines as well (Figure 6E).
Last, we calculated the ratio of S70 pBcl-2 to SOD1 for P1-P20 and correlated it with the respective prognosis of each lymphoma patient (supplemental Figure 7). Interestingly, we found that in all 5 primary lymphomas with S70pBcl-2:SOD1 ratio greater than 1, the patient had a poor prognosis and suffered from a relapse, whereas this was true in only 3 (of 11) patients with a ratio lower than 1 (Figure 6B,F). These data highlight the potential for using both S70pBcl-2 and SOD1 as prognostic markers in clinical lymphomas. In summary, our study supports the hypothesis that O2−/ONOO− production stimulates B56δY289 nitration leading to an increase in S70 Bcl-2 phosphorylation that in turn promotes tumor chemoresistance.
Discussion
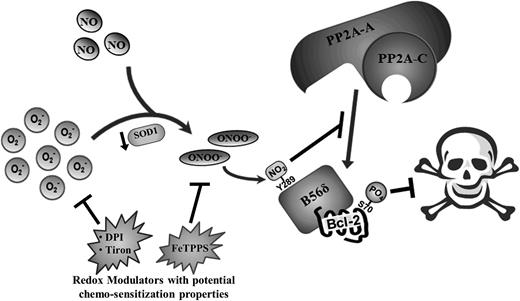
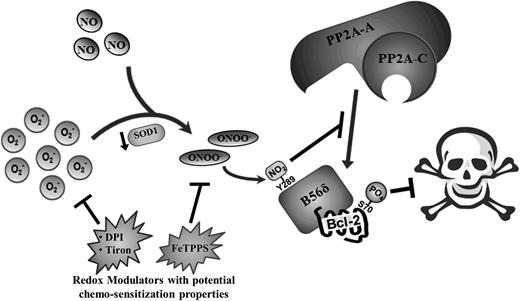
ROS-governed cellular processes are highly intricate in nature, often involving a well-orchestrated series of events that necessitate the tight regulation of numerous parameters. Likewise, ROS-dependent cell fate processes require a delicate balance of different intracellular ROS species, deregulation of which often leads to uncontrolled cell proliferation and cancer. Here, we describe a novel mechanism whereby an increase in intracellular O2− induced by either pharmacological inhibition or genetic knockdown of SOD1 promotes chemoresistance by augmenting the phosphorylation (and activation) of Bcl-2 at S70. By preventing SOD1-mediated conversion of O2− to H2O2, O2− could react more readily with NO to form ONOO−. This led to the inhibitory nitration of the Bcl-2 phosphatase, PP2AB56δ, and the consequential increase in S70pBcl-2 level. Moreover, we identified a nitration-prone tyrosine residue on the B56δ regulatory subunit of PP2A (B56δY289) that is also a known interacting site between B56δ and the A scaffolding subunit of PP2A. The nitration of the tyrosine residue impeded the association between the catalytic core and the B56δ-Bcl-2 complex and inhibited the recruitment of the AC core to the mitochondria. As a result, the dephosphorylation of Bcl-2 at S70 was inhibited, and the accumulation of phosphorylation-activated Bcl-2 ensued. These findings reveal a novel mechanism that enables cancer cells to harness the pro-survival properties of O2− to acquire a resistant phenotype (Figure 7).
Schematic summary of molecular events underlying O2−-mediated induction of S70pBcl-2 upon the downregulation of SOD1 in tumor cells. Augmented level of intracellular O2− as a result of SOD1 inhibition/downregulation could lead to an increase in the formation of ONOO− and the eventual tyrosine nitration of B56δ. This in turn inhibits the recruitment of PP2A-AC core subunits to Bcl-2, resulting in the accumulation of S70-phosphorylated Bcl-2 and the further inhibition of apoptotic stimuli. Redox modulators such as DPI, tiron, and FeTPPS may harbor the potential as chemosensitization agents by suppressing the levels of O2− or ONOO− in tumor cells.
Schematic summary of molecular events underlying O2−-mediated induction of S70pBcl-2 upon the downregulation of SOD1 in tumor cells. Augmented level of intracellular O2− as a result of SOD1 inhibition/downregulation could lead to an increase in the formation of ONOO− and the eventual tyrosine nitration of B56δ. This in turn inhibits the recruitment of PP2A-AC core subunits to Bcl-2, resulting in the accumulation of S70-phosphorylated Bcl-2 and the further inhibition of apoptotic stimuli. Redox modulators such as DPI, tiron, and FeTPPS may harbor the potential as chemosensitization agents by suppressing the levels of O2− or ONOO− in tumor cells.
The regulatory role of ROS in cell death and proliferation processes is akin a double-edged sword. Although a mild pro-oxidant state dominated by O2− anions creates an intracellular milieu that favors cell survival,19,20,32 excessive production of intracellular ROS triggers cell death via the oxidative activation of various death effectors.33 There is a dynamic interplay among the various ROS in the regulation of cell death processes. For example, we found that a tilt in cellular redox equilibrium in favor of O2− anions facilitated the formation of ONOO− via the radical fusion of O2− and NO. The resultant modest increase in ONOO− drives nitrosative modification specifically of nitration-labile tyrosine residues. The consequences of increased ONOO− are dose-dependent; low or moderate levels of ONOO− will only affect nitration-sensitive targets, whereas higher levels of ONOO− cause widespread nitration of less reactive targets as well. In fact, more is not always better: others have found that high levels of exogenous ONOO− (>75 μM) can nitrate and activate the catalytic subunit of PP2A.34,35 This could account for the biphasic effect of ONOO− observed in Figure 4C and supplemental Figure 4D, where initial increase in S70pBcl-2 and cell viability at low doses of ONOO− was followed by falling Bcl-2 phosphorylation and cell death at high-dose ONOO−.
O2−-induced chemoresistance may provide the rationale for a chemotherapeutic strategy. S70 Bcl-2 phosphorylation predicts chemoresistance.9,36 In these cases where Bcl-2 is activated by O2−, suppression of O2− might enhance the efficacy of conventional cytotoxic agents. Indeed, the idea of redox modulation as a chemotherapeutic strategy has been suggested by studies demonstrating that O2− suppressing agents such as DPI and Tempol (SOD1 mimetics) can bypass the antiapoptotic activity of Bcl-2 and sensitize tumors to drug-induced apoptosis.13,37 Pharmacological strategies that involve the suppression of intracellular O2− level may be included in the future in the design and development of effective combination therapies.
The identification of O2−-driven nitration of PP2AB56δ and changes in S70pBcl-2 may also provide valuable clinical biomarkers of redox-driven neoplastic processes. The marked inverse correlation between the abundance of SOD1 and the amount of both S70pBcl-2 and B56δ nitration in clinical lymphoma biopsies is a potential biological signature that could be exploited for prognostic and therapeutic purposes. For instance, the detection of lymphomas with low SOD1 but high S70pBcl-2 and nitrated B56δ might indicate greater drug resistance, and suggest the inclusion of redox modulators in the chemotherapeutic regime. Therefore, our findings could provide the basis of a novel approach for stratifying clinical lymphomas based on tumor aggressiveness and resistance to chemotherapy.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
The authors thank Dr Jayshree Hirpara, Dr Goh Boon Cher, and Ms Goh Han Lee for technical assistance on the primary lymphoma samples and Mr Stephen Chong for the synthesis of the Bcl-2 mutant plasmids.
This work is supported by grants from the National Medical Research Council of Singapore (grant numbers NPRC10/NM06M and NMRC/1241).
Authorship
Contribution: I.C.C.L. designed and performed the experiments, conceived the study, analyzed the data, and wrote the manuscript; T.L. provided the clinical materials and surgical specimen; Y.H. assisted in the prognostic scoring of lymphoma biopsies; D.M.V. provided anti-B56δ and anti-PP2A-C antibodies as well as the HA-tagged B56 subunit plasmid constructs, provided critical comments, and wrote the manuscript; and S.P. is the lead principal investigator, provided funding for the study, conceived the study, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shazib Pervaiz, Department of Physiology, Yong Loo Lin School of Medicine, National University of Singapore, 2 Medical Dr, MD9 #01-05, Singapore 117597; e-mail: phssp@nus.edu.sg.

![Figure 1. O2− promotes chemoresistance through the induction of S70 Bcl-2 phosphorylation. (A) Bcl-2 phosphorylation status was examined in Jurkat cells treated with increasing doses of DDC for 4 hours. A total of 400 μM was selected as the working dose for subsequent experiments. (B) Western blot of Jurkat cells after genetic silencing of SOD1 using 4 independent siRNA sequences (denoted as siSOD1-A, B, C, and D). (C) S70pBcl-2 level was assessed in Jurkat cells transfected with vector (EV) or SOD1 plasmids. (D) Immunoblot of Jurkat cells treated with bovine SOD1 (bSOD1). bSOD1 appeared as faster migrating band under human SOD1. (E) Jurkat cells were pretreated for 1 hour with tiron, followed by 4 hours with DDC, and then lysates were immunoblotted for S70pBcl-2. (F) MTT cell viability assay of Jurkat cells pretreated with DDC (1 hour) followed by treatment with the indicated doses of chemotherapeutic agents (5FU, cisplatin, etoposide [Eto], and doxorubicin [Doxo]) for 18 hours. (G) MTT assay of Eto- or Doxo-treated Jurkat cells after knockdown of SOD1 using siSOD1A, B, or C. (H) MTT assay of 5FU- or cisplatin-treated Jurkat cells after pcDNA-SOD1 transfection or after the inhibition of reduced NADP oxidase-mediated O2− production via DPI (2.5 μM) pretreatment. (I) MTT assay of Jurkat cells expressing wild-type Bcl-2, S70A Bcl-2, or S70E Bcl-2 mutant after 18 hours of Eto treatment.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/14/10.1182_blood-2014-03-563296/4/m_2223f1.jpeg?Expires=1766008756&Signature=Pt5Dx5xIxc6LWdCDSbWICpg1-DGQeovBubyqDdK0OnCqxO7ntzF6bIy0ixAIBi6RWIBfTpvkWt9U9C-9pm0NKw2FxzZmDlLbBeEbxjiX6sNUI28koxw2DY50qJsLMSEQ2wpJosADhpF3FEw7Uzn2zmUUZL84vqehEB5psLm74n1G~KtnoZcJTEyNc8hLZkHzMV5RygSMdHZ3-RDIYHag-2EcrUcjDpkMZS0xmWvOfOY3W5s4F~1BCq7d5-0Solh95xSq17mQgDBqTWyzWa44j~vWokPWGWbu34PuoHxq81-FPOxtWWHT1j5jIIo8Pl4xFCLwgY0oJkwpGfvrSsdwMQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. O2− inhibits PP2A-mediated dephosphorylation of Bcl-2 at S70. (A-C) Lysates from DDC-treated Jurkat, Hela, and MDA231 were immunoprecipitated (IP) and immunoblotted (IB) using the indicated antibodies. Input refers to whole cell lysates that are not subjected to IP procedures. Immunoglobulin G (IgG) refers to untreated lysates that were incubated with nonspecific IgG primary antibodies. (D) Mitochondria isolated from MDA231 or Jurkat cells were incubated with proteinase K for 25 minutes followed by lysis and IB for the indicated proteins. As a control, incubation of mitochondria with Triton-X100 resulted in the digestion of SOD2 (matrix protein) and COX Va (inner mitochondrial membrane [IMM] protein). (E) Mitochondrial/cytosolic fractions from DDC-treated Jurkat or MDA231 cells (with or without tiron pretreatment) were IB for the indicated proteins. SOD1 was employed as a cytosolic marker, whereas prohibitin, an IMM protein, served as a mitochondrial marker. (F) Confocal fluorescence imaging studies of DDC-treated MDA231 cells. Colocalization of Bcl-2 (red) and PP2A-C (green) appears as yellow punctate (red arrows). (G) Confocal imaging of Jurkat cells 48 hours after silencing of SOD1. Selected cells were enlarged for greater clarity.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/14/10.1182_blood-2014-03-563296/4/m_2223f2.jpeg?Expires=1766008756&Signature=m9KuHM4LJqz1oJdVyd~919XumBS-G9lJzMS-pHu2IpTow9nQRTiX~jiDe9aBv~~qwPiqDCFJwVy9UogzIQWoBg47w4dRM2wZ7O5otYnhkXeShoJh3xjWkRLwZrCJoWh0BXmbzqxEp2VKa6xIvhpx31XSoGQNCuULqrcsk-Ofm4fPmqkFqNdYRQfp1QGrjG4YkFWc~HrdBwKOg9JZiHswkGYslG5SpjD~IViIKVnhr~PrdYliJ3xI5Wj4PKcHF~J7tsv4voUq-umDBngHi7zemxs6H0nBs3LkJ1M-pAOeFS4QFM1YYxiEfZtAK1Nz4aKrqoiYb3PCnwcn2Ltu1-3YbA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 1. O2− promotes chemoresistance through the induction of S70 Bcl-2 phosphorylation. (A) Bcl-2 phosphorylation status was examined in Jurkat cells treated with increasing doses of DDC for 4 hours. A total of 400 μM was selected as the working dose for subsequent experiments. (B) Western blot of Jurkat cells after genetic silencing of SOD1 using 4 independent siRNA sequences (denoted as siSOD1-A, B, C, and D). (C) S70pBcl-2 level was assessed in Jurkat cells transfected with vector (EV) or SOD1 plasmids. (D) Immunoblot of Jurkat cells treated with bovine SOD1 (bSOD1). bSOD1 appeared as faster migrating band under human SOD1. (E) Jurkat cells were pretreated for 1 hour with tiron, followed by 4 hours with DDC, and then lysates were immunoblotted for S70pBcl-2. (F) MTT cell viability assay of Jurkat cells pretreated with DDC (1 hour) followed by treatment with the indicated doses of chemotherapeutic agents (5FU, cisplatin, etoposide [Eto], and doxorubicin [Doxo]) for 18 hours. (G) MTT assay of Eto- or Doxo-treated Jurkat cells after knockdown of SOD1 using siSOD1A, B, or C. (H) MTT assay of 5FU- or cisplatin-treated Jurkat cells after pcDNA-SOD1 transfection or after the inhibition of reduced NADP oxidase-mediated O2− production via DPI (2.5 μM) pretreatment. (I) MTT assay of Jurkat cells expressing wild-type Bcl-2, S70A Bcl-2, or S70E Bcl-2 mutant after 18 hours of Eto treatment.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/14/10.1182_blood-2014-03-563296/4/m_2223f1.jpeg?Expires=1766147621&Signature=qPmnfADqiPVbP7zKIn-HMZN8XXsSegfG1Yc7QJc8mue-JKyaxB8mSXtwcgkXG~vAiFGnLjQl118Iz0s5osQO94RQw8TuVpyOooOiHbxn4LX~bsZw~Wgt2X12akKGqTUelAtjBLKqEywlmAmpgRdJ~fGEUUB9hIAAdKB8kFckc9izuTymhefTCWQXLXw3OWHZfTUBEnDACInaXvuxm7416NDPudvY1tvqiPXtiJHM2P9P8vIkDXGodRjB2MJzHk0cq7~YkqOBi-VFBigK1AUPqfrtrBaTIISApe9J3EWIWHizxgc88aREiUDzGFwoResJZVq8eEsl41XeJQqt8qGY8Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. O2− inhibits PP2A-mediated dephosphorylation of Bcl-2 at S70. (A-C) Lysates from DDC-treated Jurkat, Hela, and MDA231 were immunoprecipitated (IP) and immunoblotted (IB) using the indicated antibodies. Input refers to whole cell lysates that are not subjected to IP procedures. Immunoglobulin G (IgG) refers to untreated lysates that were incubated with nonspecific IgG primary antibodies. (D) Mitochondria isolated from MDA231 or Jurkat cells were incubated with proteinase K for 25 minutes followed by lysis and IB for the indicated proteins. As a control, incubation of mitochondria with Triton-X100 resulted in the digestion of SOD2 (matrix protein) and COX Va (inner mitochondrial membrane [IMM] protein). (E) Mitochondrial/cytosolic fractions from DDC-treated Jurkat or MDA231 cells (with or without tiron pretreatment) were IB for the indicated proteins. SOD1 was employed as a cytosolic marker, whereas prohibitin, an IMM protein, served as a mitochondrial marker. (F) Confocal fluorescence imaging studies of DDC-treated MDA231 cells. Colocalization of Bcl-2 (red) and PP2A-C (green) appears as yellow punctate (red arrows). (G) Confocal imaging of Jurkat cells 48 hours after silencing of SOD1. Selected cells were enlarged for greater clarity.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/14/10.1182_blood-2014-03-563296/4/m_2223f2.jpeg?Expires=1766147621&Signature=0BijFWHRzKAaLWj5wHKA7mGe~pVIHgDwpKkCWcjILQTJRG-N0h2eLmURp437a8~40nwZhNWzYyv6JTqgDurnFx2LJs4BHxUsRq-H3SCL4NSu5cTC~t1L1YAprD-12aIK-xcFylRJjuets0CscxHlLRyf0cOd9FLdkRmRh~tl3zk-T0QjLWccqu78Vil74crFxRCtjLa2lNyiruY2kSk2pKQRh6h0dGfEDCrqZH9EArwnJXDTRrjEJ3XXvM5ydbIKhwiSfo4yZtHqc25Kg~PijLrs0ORgBF8v597FjnL0izvycyjL8t3Bodpbhf3QluUdECGOe0tkfVblzEpw4ngH6Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)