Abstract
Objective: Although Hedgehog (Hh) and Wnt signaling pathways are known to play a role in cancer cell growth, their specific effects on leukemia cells are not fully understood. Previously we reported that the Hh inhibitor, cyclopamine, and the Wnt inhibitor, quercetin, suppressed the growth of leukemia cells. However, a specific inhibitory role of these chemical compounds on Hh and Wnt signaling could not be confirmed since these compounds also have off-target effects. Therefore the effects of specific inhibition of Hh and Wnt signaling pathways must be investigated in detail. In our previous study, we reported that small interfering RNA (siRNA)-mediated knockdown of NOTCH1 and NOTCH2 suppressed growth of T-ALL cell lines. In this context, the relationship between Hh, Wnt, Notch and mTOR signaling; known to regulate stemness; has not been fully elucidated. To address these questions, the effects of siRNA-mediated knockdown of GLI1 and CTNNB1 (Catenin beta 1), which respectively mediate Hh and Wnt signaling, on cell proliferation as well as Notch and mTOR signaling were studied in AML and T-ALL cell lines.
Methods: Two AML cell lines (NB4 and THP-1) and 2 T-ALL cell lines (DND41 and Jurkat) were used in this study. siRNAs targeting GLI1 (siGL), CTNNB1 (siCT), NOTCH1 (siN1) or control siRNA were transfected into cells by using the pipette tip chamber-based electroporation system. The effects of siGL and siCT transfection on cell proliferation were examined using a colorimetric WST-8 assay and by observing cytospin preparations of the harvested cells. The effects on mRNA and protein expression were examined by quantitative RT-PCR and immunoblotting, respectively.
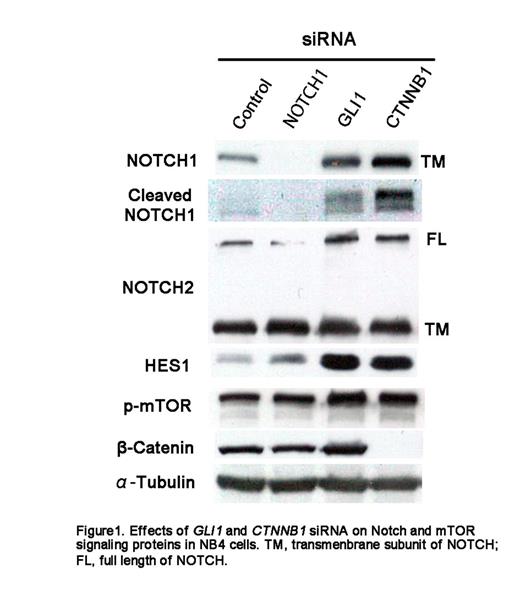
Results: Transfection with siGL and siCT selectively suppressed the expression of GLI1 and CTNNB1, respectively. siRNA treatment did not lead to any significant effects on cell proliferation or morphology in all tested cell lines. In NB4 cells, GLI1 as well as CTNNB1 knockdown increased the level of NOTCH1, the cleaved NOTCH1 fragment (active form of NOTCH1), HES1, and phosphorylated mTOR protein. In the other cell lines, the expression of these proteins was not significantly altered by siGL and siCT.
Discussion: siRNA-mediated knockdown experiments suggested that Hh and Wnt signaling had insignificant effect on the proliferation of the leukemia cell lines used in this study. We report a novel interaction, namely, the activation of Notch and mTOR signaling by the suppression of Hh and Wnt signaling in NB4 cells. The molecular mechanism and significance of this phenomenon as well as its translation to other cell lines needs further examination. We propose that this finding must be taken into account when Hh- and Wnt-targeted therapy against leukemia are developed.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.