Complement, besides its involvement in eliminating microbes, participates in such diverse processes as synapse maturation, clearance of immune complexes, angiogenesis and tissue regeneration. Delicate balance between complement activation and regulation contributes to complement’s role in physiology. Any trigger that tips this balance can induce self-attack. Atypical hemolytic uremic syndrome (aHUS) is a systemic disease characterized by non-immune hemolytic anemia, thrombocytopenia, and renal impairment. In over 50% of cases, aHUS is known to be caused by uncontrolled activation of the complements.
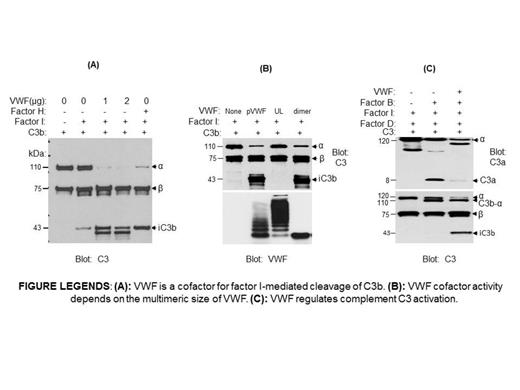
Von Willebrand factor (VWF) is a large multimeric glycoprotein that plays an important role in stopping the escape of blood from vessels following vascular injury. VWF and VWF cleavage enzyme ADAMTS13 gene defects have been identified in patients with aHUS, raising the possibility that VWF could have contributed to complement regulation. To examine the role of VWF in complement activation, we investigated whether VWF functions as a cofactor for FI-mediated C3b cleavage through in vitro assay. We found that C3b binds to VWF. In the presence of plasma-purified VWF (pVWF), FI cleaves C3b to 68kD and 43kD degradation products (iC3b) (Figure (A)). VWF alone, or FI alone, did not have any effect on C3b cleavage. C3b, not C3 or iC3b, was the mainly substrate for FI/VWF proteolysis. Increasing VWF concentration or prolonging the incubation time with VWF enhanced FI-mediated C3b cleavage. To remove the possibility that another plasma protein co-purified with pVWF that affects our results, we used recombinant VWF dimers purified from human embryonic kidney (HEK) 293 cell expressing VWF-Dpro cDNA, and detected a similar cofactor activity (Figure (B)).To investigate whether the size of VWF multimers have any effect on C3b cleavage, we compared the cofactor activity of pVWF, recombinant VWF dimers, and ULVWF multimers. While pVWF and dimer VWF enhanced C3b cleavage by FI, ULVWF did not have any effect on C3b cleavage (Figure (B)). In plasma, complement proteins Factor B and Factor D coexist with the inhibitory protein FI. To investigate the effect of VWF on complement activity in the presence of both pro-activation and inhibitory complement proteins, we incubated C3, FB, FD, and FI with VWF. In the presence of FB and FD, C3 be activated and resulted in the generation of C3a and C3b. Addition of recombinant VWF put a brake on complement activation and shifted C3 toward the generation of iC3b (Figure (C)). We conclude that normal plasma VWF, function as a cofactor, prevents complement activation through steers the complement pathway toward the generation of inactive iC3b. ULVWF multimers, as are present in patients with thrombotic microangiopathy, lack an inhibitory effect on complement and permit complement activation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.