Abstract
Background: CD53, a member of the tetraspanin family of proteins, broadly expressed in hematopoietic cells, is implicated in leukocyte activation and cell survival responses to stress conditions. In a recent genome linkage scan, the single nucleotide variation, rs6679497 (C>G), in the intron of the CD53 gene was identified as an important regulator of TNF release from innate immune cells in response to inflammatory stimuli. (Bos SD: Eur J Hum Genet. 2010). Tumor necrosis factor alpha (TNF-ƒ¿) which is released pretransplant due to irradiation and cytotoxic preconditioning regimens, has been implicated in transplant complications.
Methods: To assess the impact of rs6679497 variation of the CD53 gene, on transplant outcomes, we evaluated 322 of patients with hematologic malignancies transplanted through the Japan Marrow Donor Program. The functional relevance of the rs6679497 variation was investigated in a whole blood culture system using samples from healthy individuals. To investigate the effect of rs6679497 on mRNA transcription activity we performed allele-specific quantitative RT-PCR with a TaqMan probe of rs6679497 using cDNA from in vitro activated leukocytes from healthy donors with heterozygous genotypes.
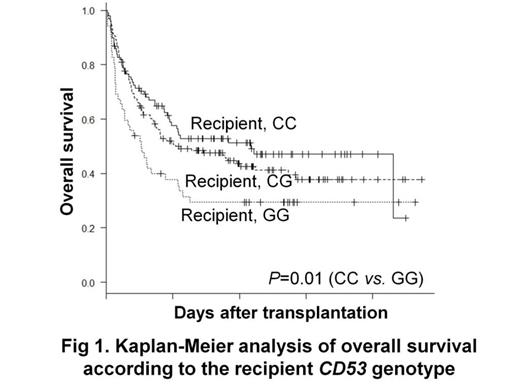
Results: There was a significantly lower overall survival in the recipients with the GG genotype in the univariate analysis (29% vs. 53% at 5-y; P=0.01) and multivariate analysis (relative risk=1.97; 95% confidence interval, 1.22-3.18; P=0.01, Figure 1). The donors s6679497 genotypes did not significant influence the transplant outcomes. In the allele-specific quantitative PCR, the mean ratio (G allele transcripts vs. C allele transcripts) was 1.5 which is higher than that of DNA amplicons, suggesting that the G allele has higher transcriptional activity than the C allele. Using the TF-SEARCH online tool, which predict putative transcription factor binding sites, the genomic region containing the G allele of s6679497 was found to create a novel consensus sequence corresponding to the putative binding element of GATA-1. In vitro stimulated leucocytes from healthy individuals possessing the G allele secreted higher levels of TNF-ƒ¿ than those without the G allele. Consistent with these results, flow cytometry studies revealed that monocytes from donors possessing the G allele express higher levels of CD53 on their surface (p=0.02) which may account for their higher TNF-ƒ¿ secretion. These findings substantiate the functional relevance of the rs6679497 variation, and indicate that the increased TNF-ƒ¿ secretion by individuals with the G allele likely account for their worse OS.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.