Abstract
Background: Erythropoietin stimulating agents (ESAs), have been readily used to treat chemotherapy related anemia in cancer patients since 2003. ESAs are a useful substitute for blood transfusions for these patients. However, there were unacknowledged toxicities for ESAs when they were first administered. In March 2007, these toxicities were acknowledged by the FDA when a black box warning was issued for ESAs in these patients. This study aims to observe ESA utilization trends along with VTEs to determine the effect of the black box warning.
Methods: The 2002-2010 merged South Carolina Medicaid- Cancer Registry dataset with Medicaid claims up to 2012 was used to determine the effect of the FDA black box warning. Breast, non-small cell lung and colorectal patients were determined from the cancer registry. Of these, those who had chemotherapy were identified. ESA users were identified from medical and pharmacy claims. The final sample contained those who had their first ESA claim after chemotherapy and who were not dual enrolled in Medicare.
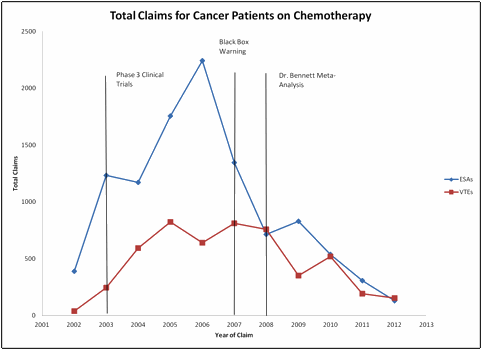
Results: Of the 1,645 cancer patients with chemotherapy, 1,310 (80%) had at least one ESA claim. Total ESA claims by year showed a peak in 2006 at 2,243 claims followed by a decrease to 1,347 in 2007 and 714 in 2006. A month by month breakdown of ESA claims showed the peak in May 2006 at 250 and eventually decreasing to 7 in December 2012. Total VTE events for these patients showed a peak in 2007 at 811 followed by a steep decline to 351 in 2009 and 154 in 2012.
Conclusion: The FDA black box warning may have been effective in reducing ESA utilization. There is also the potential delayed response to the clinical trial data for VTEs and ESA utilization. The meta-analysis in 2008 by Dr. Bennett also seems to have had an impact on reducing ESA utilization and VTE events.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.