Abstract
Synucleins (including α-, β- and γ-synucleins) are a group of proteins that are highly expressed in the central nervous system. Alpha-synuclein, in particular, has been implicated in the pathogenesis of neurodegenerative disorders known as synucleinopathies. The function of these proteins in other organ systems is largely unknown. Some studies have demonstrated expression of α-synuclein on peripheral blood mononuclear cells (PBMC), including B and T lymphocytes, NK cells and monocytes; and its expression has been shown to be higher in PBMCs of individuals with Parkinson’s compared to healthy controls. We have recently shown that α-synuclein-deficiency is associated with marked defect in development of mature B and T lymphocytes. In particular, we showed enhanced negative selection of developing thymic T cells in the absence α-synuclein. Furthermore, we demonstrated that α-synuclein-deficiency is associated with an impaired IgG response to T cell-dependent antigens.
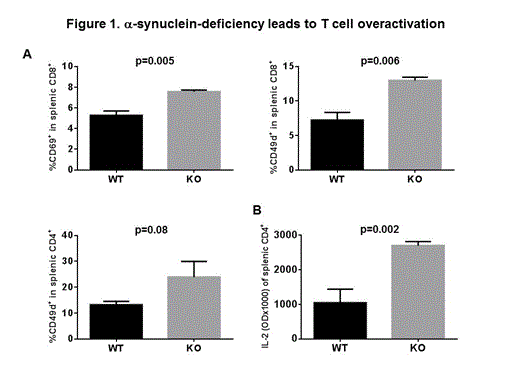
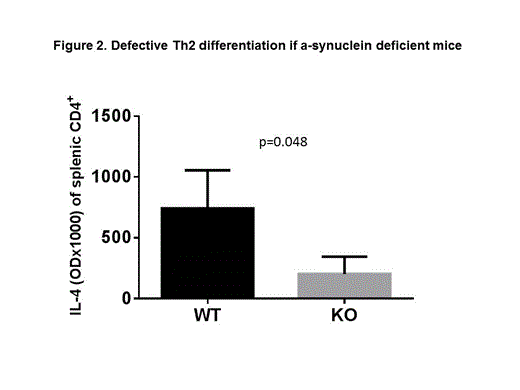
Here we used age and sex-matched α-synuclein knock-out (KO) and wild type (WT) mice to further investigate the lineage differentiation and activation of T cells. We found that few splenic T cells that develop in KO mice contain a higher percentage of CD8+ T cell expressing early activation markers CD69 (7.6 ± 0.09 for KO vs. 5.3 ± 0.23 for WT, p=0.005, figure 1A) and CD49d (12.97 ± 0.3 for KO vs. 7.32 ± 0.6 for WT, p=0.006, figure 1A). A similar trend was noted for CD4+ CD49d+ T cells, although the difference did not reach statistical significance (23.90 ± 3.48 for KO vs.13.23 ± 0.73 for WT, p=0.086, figure 1A). No difference was noted in the expression of late activation marker CD44, and lymph node homing marker CD62L. This was associated with significantly increased IL-2 production from KO CD4+ T cells (OD 2.70 ± 0.12 for KO vs.1.05 ± 0.39 for WT, p=0.002, figure 1B) and a trend for increased IFN-γ production from KO CD4+ T cells (OD 2.89 ± 0.33 for KO vs.2.12 ± 0.59 for WT, p=0.12) after in vitro activation with anti-CD3/anti-CD28 beads. Interestingly, In vitro activation of splenic CD4+ T cells resulted in significantly reduced IL-4 production from KO T cells (OD 0.20 ± 0.14 for KO, vs. 0.74 ± 0.31 for WT, p=0.05, Figure 2) suggesting a defective Th2 differentiation in KO CD4+ T cells.
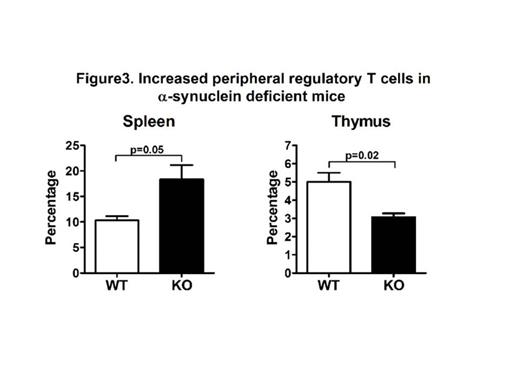
Further flow cytometric analysis of T cells showed that while thymic Foxp3+ CD4+ regulatory T cells are significantly reduced in KO mice (3.07 ±0.35 for KO vs. 5.00 ± 0.87 for WT, p=0.02, Figure 3), the percentage of splenic Foxp3+ CD4+ T cells is higher in the KO mice compared to WT mice (18.33 ± 4.90 for KO vs.10.33 ± 1.39 for WT, p=0.05, Figure 3). No difference was noted among NK cells from KO and WT mice.
In summary, we demonstrate a role for α-synuclein in lineage differentiation and function of T cells. While α-synuclein-deficiency leads to a significant defect in development of mature T cells, the small population of cells that do mature, express higher levels of early activation markers, and produce higher levels of IL-2 upon antigenic stimulation. Of interest, these cells are defective in IL-4 production. Additionally, we also show that α-synuclein-deficiency is associated with a higher percentage of peripheral CD4+ Foxp3+ T cells, a finding that might be explained by higher levels of IL-2 production by α-synuclein-deficient CD4+ T cells, although a direct effect of α-synuclein on the survival of regulatory T cells cannot be excluded. The underlying mechanism for the function α-synuclein in development and function of T cells is subject of future studies.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.