Abstract
The tumor microenvironment plays a central role in the pathogenesis of follicular lymphoma (FL) and has been shown to influence prognosis. The biological basis for this and the contribution of individual cell types however, remain unclear. In this study we compared the cellular content and structure of neoplastic follicles in FL with their normal counterparts in reactive lymph nodes (LNs). We specifically focused on follicular helper T cells (TFH) which, in normal germinal centers (GCs), form immune synapses with antigen responsive B cells triggering B cell proliferation and expression of activation induced cytidine deaminase (AID), the enzyme required for somatic hypermutation and class switch recombination. This is of relevance because off-target AID activity is thought to play a role in generating the mutations that characterize progressive FL.
A limitation of previous studies of the FL microenvironment is the use of either single parameter immunohistochemistry which fails to accurately define the complex populations of cells involved, or flow cytometry on disaggregated cells which results in the loss of architectural information. In this study we used multiparameter confocal immunofluorescent (IF) microscopy to investigate in vivo the phenotype, distribution and interaction of CD4+ T cells in FL and to determine to what extent these are similar to normal GCs.
Confocal IF microscopy was performed on multiple sections of formalin fixed paraffin embedded LN biopsy specimens from 20 patients with untreated FL, comparison was made with reactive LNs (n=5) and chronic lymphocytic leukemia (CLL) LN biopsies (n=5). Each section was stained with a combination of up to 4 simultaneously applied primary antibodies against CD3, CD4, CD20, PD1, ICOS, BCL6, AID, and Ki67, and fluorescently labelled secondary antibodies. Microscopy was performed using a Nikon TiE fluorescent microscope equipped with A1R Si Confocal imaging system; images were analyzed using NIS software.
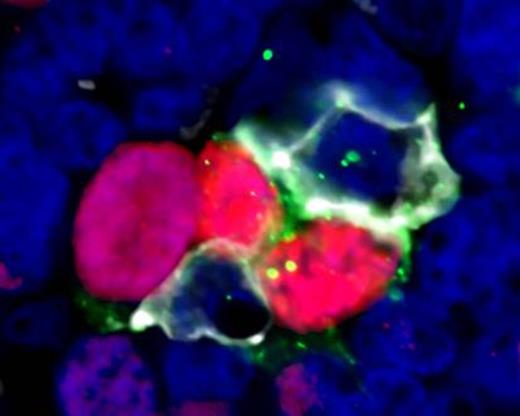
Results show that CD4+ T cells in FL are mainly located in the inter-follicular regions but they were also identified within the follicles in all cases. Combination staining with anti-CD4, PD1, and ICOS revealed that 23% (95%CI 18-27) of CD4+ T cells within follicles co-express PD1 and ICOS consistent with a TFH phenotype which is significantly higher than in inter-follicular areas where only 5% (95% CI 3-7) of CD4+ cells had this phenotype (p<0.001). PD1+ ICOS+ T cells were positive for the transcription factor BCL6, further confirming the TFH phenotype. There was no significant difference in the proportion of CD4+ cells that were TFH in FL follicles and reactive LN GCs. In CLL cases, 54% of CD4+ cells expressed PD1 but only 9% co-expressed PD1 and ICOS, significantly lower than either FL follicles or GCs (p<0.001). Automated analysis of 3D z-stacks demonstrated a very close spatial relationship between proliferating tumor cells and TFH in FL with a mean of 42% (95%CI 35-48) Ki67+ tumor cells in direct contact with TFH cells. No association was seen between the extent of co-localization and histological grade. A similar pattern of co-localization of TFH cells next to proliferating B cells was also identified in the light zones of reactive GCs. Of note, we also identified features of synapse formation between TFH cells and proliferating tumor cells; TFH cells demonstrated projections that encompass the tumor cell with distortion of the T cell nucleus and increased CD4 and PD1 expression at sites of cell contact (Figure 1). These findings were similarly present in reactive GCs. Finally, AID was expressed in proliferating GC B cells and in proliferating tumor cells in FL. AID expressing cells were found to be in close contact with PD1+ T cells in both GCs and FL.
Our findings show many parallels between the follicles of FL and normal GCs. In particular the proportion of CD4+ T cells with a TFH phenotype and their localization in direct contact with proliferating AID+ B cells were very similar. Of note, features of immune synapses were observed in both GCs and FL. Taken together, the data suggest that TFH cells have an important role in the pathogenesis of FL just as they are vital in the normal GC reaction. Interruption of this interaction is a potential therapeutic target.
High power view (x60 zoom) of follicular lymphoma showing proliferating cells in close contact with TFH cells. Ki67 (red), PD1 (white), ICOS (green), DAPI (blue)
High power view (x60 zoom) of follicular lymphoma showing proliferating cells in close contact with TFH cells. Ki67 (red), PD1 (white), ICOS (green), DAPI (blue)
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.