Abstract
Background and Objective: The Src family consists of Src, Lck (p56Lck), Fyn, Hck, Blk, Lyn, Fgr, Yes, and Yrk protein tyrosine kinases, which display cell and tissue specific expression. These non-receptor protein tyrosine kinases regulate many cellular processes including cell growth, differentiation, shape, migration, and survival. p56Lck is primarily expressed in T lymphocytes where it stimulates T cell receptor-mediated signaling to regulate thymocyte development. Similar to other SRC family members, p56Lck activity is negatively regulated by phosphorylation of Tyr505 and undergoes autophosphorylation on Tyr394 in the active state. Our previous studies showed that p56Lck activation occurred in proliferating endothelial cells exposed to cleaved high molecular weight kininogen (HKa), which induced endothelial cell apoptosis.The purpose of this study is to define the role of Lck in regulation of endothelial cell survival and angiogenesis.
Methods: Primary cultures of endothelial cells were treated with Lck siRNA or with mammalian expression vectors or lentiviral constructs containing Lck cDNA. The proliferative capacity, viability, and tube-forming ability of Lck and control endothelial cells were determined.
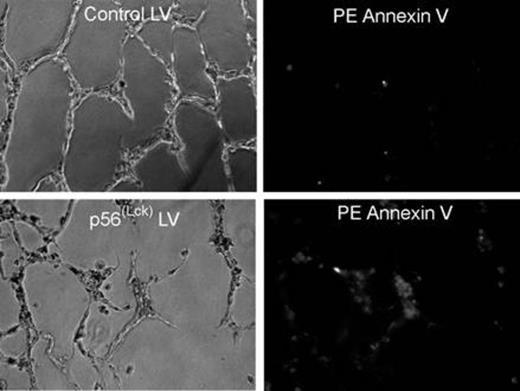
Results: Transfection of endothelial cells with Lck siRNA markedly changed their morphology and significantly enhanced their ability to proliferate in response to bFGF. In contrast, endothelial cells transfected with a Lck expression vector or transduced with a Lck-lentivirus not only failed to proliferate in response to growth factors, but also underwent apoptosis on several different extracellular matrix proteins. Finally, overexpression of Lck in endothelial cells led to impaired tube formation in a matrigel-based, in vitro angiogenesis assay; apoptosis of endothelial cells in this system was suggested by increased staining with Annexin V.
Conclusion: Taken together, these results suggest that regulation of Lck activation provides a key node in determination of endothelial cell proliferation and survival that is likely to be relevant to the regulation of angiogenesis. Moreover, they suggest that specific endothelial cell Src family kinase members may play contrasting roles in regulation of these processes. Activation of Lck may represent a potential mechanism for controlling aberrant angiogenesis in pathological conditions.
Effect of control or p56Lck-expressing lentiviral transduction on endothelial cell tube formation and induction of apoptosis.
Effect of control or p56Lck-expressing lentiviral transduction on endothelial cell tube formation and induction of apoptosis.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.