Abstract
Introduction
Recombinant FXIII (rFXIII) represents a new treatment opportunity for patients with congenital FXIII A-subunit deficiency. Monthly prophylaxis with 35 IU/kg rFXIII was shown to effectively control bleeding with an annualized bleeding rate (ABR) of 0.138 bleeds requiring treatment per patient per year and an excellent safety profile (Inbal A, et al. Blood 2012;119:5111-7). PK analysis revealed first-order elimination of rFXIII with a geometric mean half-life of 13.6 days. All patients had a mean FXIII trough activity level of >0.1 IU/mL (Kerlin B, et al.JTH 2013;11:235). The PK profile was independent of age, supporting that monthly dosing with a fixed 35 IU/Kg rFXIII regimen for all patients with FXIII A-subunit deficiency (regardless of age) was adequate for prophylaxis (Brand-Staufer B, et al. Blood 2013;122:3613. Williams M, et al. Haemophilia 2014;20:99–105). Due to the novel nature of this recombinant molecule, a phase 3b safety extension program was offered to bridge to product availability, the extensive interim results of which are analyzed here.
Methods
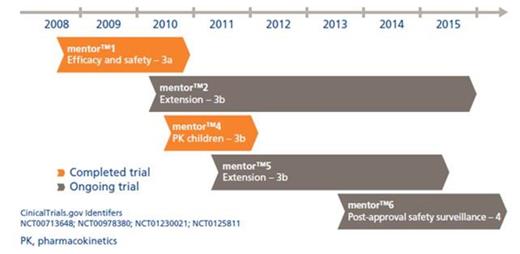
The mentorTM2 trial is an ongoing safety extension trial to the pivotal mentorTM1 trial (Figure). All patients were dosed with 35 IU/kg rFXIII every 4th week.
A planned interim analysis for mentorTM2 trial was performed (data cut-off: 31-DEC-2013).
The Berichrom® FXIII activity assay was used for measurement of FXIII activity.
Results
Sixty patients have been enrolled into mentorTM2; baseline patient demographics are presented in the Table. Of these 60, 34 patients were enrolled from the mentorTM1 trial, and 26 new patients were enrolled.
| Age, median (range) | 26 (7-77) |
| Age, mean (range) | 31 (7-77) |
| Male sex, n (%) | 38 (63) |
| Race, n (%) Black or African American American Indian or Alaska Native White Asian* Other Unknown** | 6 (10) 1 (2) 34 (57) 9 (15) 6 (10) 4 (7) |
| Body Mass Index, median (range) | 23.7 (12.8-36.9) |
| Height in cm, median (range) | 167.0 (131.0-187.5) |
| Weight in kg, median (range) | 67.5 (22.0-119.4) |
| Age, median (range) | 26 (7-77) |
| Age, mean (range) | 31 (7-77) |
| Male sex, n (%) | 38 (63) |
| Race, n (%) Black or African American American Indian or Alaska Native White Asian* Other Unknown** | 6 (10) 1 (2) 34 (57) 9 (15) 6 (10) 4 (7) |
| Body Mass Index, median (range) | 23.7 (12.8-36.9) |
| Height in cm, median (range) | 167.0 (131.0-187.5) |
| Weight in kg, median (range) | 67.5 (22.0-119.4) |
* Including 5 Japanese
** French patients are marked as unknown as per the French authorities guideline
In mentorTM2, 60 patients had 2,157 exposures (monthly dosing), corresponding to a total of 168 patient years. The ABR was 0.042 bleeds/patient/year overall, 0.012 bleeds/patient/year for spontaneous bleeds, and 0.030 bleeds/patient/year for traumatic bleeds. In total 6 patients experienced 7 bleeds requiring FXIII treatment (5 trauma-induced and 2 spontaneous). Of these 7 bleeds, 1 trauma-induced muscular bleed was treated with rFXIII with excellent hemostatic response. No intracranial, internal organ or severe gastrointestinal bleeds occurred during the trial period.
Mean FXIII trough levels were greater than 0.10 IU/mL in all patients.
No thromboembolic events, fatal adverse events or adverse events leading to withdrawal were reported. Serious adverse events were few (16 events in 10 patients) and were evaluated by the investigators as unlikely related to trial drug. No anti-rFXIII antibodies were detected.
Discussion
Prophylaxis of bleeding of patients with congenital FXIII A-subunit deficiency with rFXIII in the mentor™ trials program has demonstrated very effective bleed control, with an excellent safety profile. The ABR in the ongoing mentorTM2 safety extension trial was 0.042, which is lower than the rate of 0.138 seen in the mentor™1 pivotal study. A bleeding rate of 0.042 corresponds to an average patient having 1 bleed approximately every 24 years. One patient who had a traumatic breakthrough bleed was treated with rFXIII with excellent outcome. These efficacy data, combined with comprehensive PK- and safety data, represent the largest data collection in congenital FXIII A-subunit deficiency in the world, and provide extensive evidence for the safety and efficacy of monthly prophylaxis with 35 IU/kg rFXIII.
Fukutake:Novo Nordisk: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Baxter: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Bayer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Biogen Idec: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kaketsuken: Consultancy, Honoraria, Research Funding, Speakers Bureau; CSL Behring: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SRL, Inc: Consultancy; LSI Medience Corporation: Consultancy, Honoraria, Speakers Bureau; Chugai Pharm: Honoraria, Membership on an entity's Board of Directors or advisory committees; TORII PHARMACEUTICAL CO., LTD.: Honoraria; Sekisui Medical CO., LTD: Honoraria, Research Funding, Speakers Bureau. Carcao:Baxter, Bayer, Biogen, Novo Nordisk, Pfizer, CSL Behring, Octapaharma : Honoraria, Research Funding, Speakers Bureau. Kerlin:Bayer HealthCare US: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees. Oldenburg:Baxter: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Biogen Idec: Honoraria, Membership on an entity's Board of Directors or advisory committees; Biotest: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; CSL Behring: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Grifols: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novo Nordisk: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Octapharma: Honoraria, Membership on an entity's Board of Directors or advisory committees; Swedish Orphan Biovitrum: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Rosholm:Novo Nordisk: Employment, Equity Ownership. Garly:Novo Nordisk: Employment, Equity Ownership. Nugent:Novo Nordisk: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract