Abstract
Peroxiredoxins (PRDXs) are abundant antioxidant enzymes. In mammals there are six PRDXs. Among them, four (PRDXs 1-4) are functional dimers. PRDXs are oxidoreductases that scavenge peroxides with reducing equivalents from thioredoxin-thioredoxin reductase system. PRDXs function as a defense against redox stress but their role far exceeds peroxides removal. They directly support the activation of several protein tyrosine phosphatases thus suppressing kinase-mediated signaling. PRDXs are usually highly expressed in tumor cells, but there are cases of their epigenetic downregulation. Depending on a particular experimental model and the stage of tumor development, PRDXs can either suppress or support tumor cell growth. The role of peroxiredoxins in B-cell derived tumors has not been studied so far.
We have used two Burkitt's lymphoma cell lines: Raji and Ramos. To isolate protein targets for a novel small molecule peptidomimetic inhibitor (Klossowski, 2012, J Med Chem) with antitumor activity we have used biotin affinity probe labelling approach and mass spectrometry. Covalent crosslinking of PRDXs dimers as well as the activation of MAPK signaling were investigated by Western blotting. We employed quantitative real time PCR and Western blotting to analyze the expression of PRDX1 and PRDX2 in a range of human leukemia and lymphoma cell lines at mRNA and protein levels, respectively. To study the role of PRDX1 and PRDX2 in lymphoma cells, we have employed the shRNA-mediated knockdown approach. To evaluate cell viability and proliferation rate, viable cells were counted in a hemocytometer or, alternatively, propidium iodide-negative cells were counted using BD Accuri flow cytometer. To study cell cycle we analyzed DNA content by propidium iodide staining followed by flow cytometry and evaluated levels of cell cycle-associated markers, e.g. p21 and cyclin E2 using Western blotting. To detect apoptosis we evaluated the levels of cleaved caspase-3, caspase-9, and PARP with Western blotting.
We have found that in human Burkitt's lymphoma cell lines, a novel peptidomimetic inhibitor with antitumor activity targets dimeric 2-cystein peroxiredoxins. In Raji and Ramos cells, the inhibitor covalently crosslinks PRDX dimers, triggers MAPK activation, redox stress, cell cycle arrest in G1 phase, and apoptosis. Next, we have analyzed the levels of PRDX1 and PRDX2 in a range of human leukemia and lymphoma cell lines, and found them upregulated, as compared to normal B cells. To further study the role of PRDX1 and PRDX2 in lymphoma cells, we have reduced the level of PRDX1 in Raji cells expressing significant levels of PRDX2 as well as in a subclone of Raji cells, which does not express PRDX2. The effects of PRDX1 downregulation strongly depend on the level of PRDX2 expression. In the cells which express PRDX2 a decrease in PRDX1 expression results in the suppression of cell proliferation and cell cycle arrest in G1 phase. In Raji cells, which do not express PRDX2, the downregulation of PRDX1 entirely inhibits cell growth and induces apoptosis.
We show that in lymphoma cell lines dimeric peroxiredoxins are targets of a small molecule peptidomimetic inhibitor. Our findings indicate that PRDX1 and PRDX2 support lymphoma cell proliferation and survival. Moreover, they can substitute each other, at least to some extent, suggesting overlapping functions of these two cytoplasmic PRDXs in lymphoma cells.
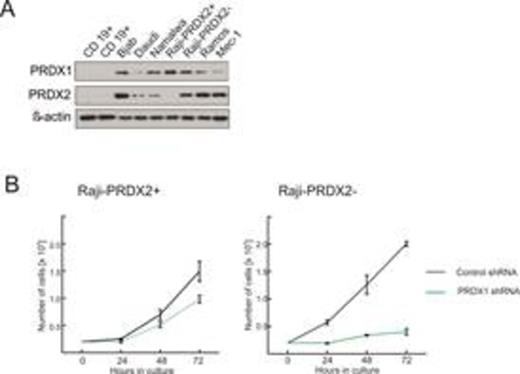
(A) Protein level of PRDX1 and PRDX2 in a panel of Burkitt's lymphoma (Bjab, Daudi, Namalwa, Raji, Ramos) and B-CLL (Mec1) cell lines as compared to normal B cells isolated from healthy donors using magnetic beads (CD19 positive selection, EasySep). (B) Effects of PRDX1 downregulation by shRNA on proliferation and survival of Raji cells. Raji cells, which do express PRDX2 (Raji-PRDX2+) and the subclone of Raji cells which do not express PRDX2 (Raji-PRDX2-), were transduced with lentiviruses encoding PRDX1-specific shRNA and control, non-targeting shRNA and subjected to selection with puromycin. The number of viable cells was evaluated 24, 48, and 72 hours post selection.
(A) Protein level of PRDX1 and PRDX2 in a panel of Burkitt's lymphoma (Bjab, Daudi, Namalwa, Raji, Ramos) and B-CLL (Mec1) cell lines as compared to normal B cells isolated from healthy donors using magnetic beads (CD19 positive selection, EasySep). (B) Effects of PRDX1 downregulation by shRNA on proliferation and survival of Raji cells. Raji cells, which do express PRDX2 (Raji-PRDX2+) and the subclone of Raji cells which do not express PRDX2 (Raji-PRDX2-), were transduced with lentiviruses encoding PRDX1-specific shRNA and control, non-targeting shRNA and subjected to selection with puromycin. The number of viable cells was evaluated 24, 48, and 72 hours post selection.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.