Abstract
Despite impressive cure rates obtained with modern chemotherapy, the optimal therapeutic strategy in advanced classic Hodgkin Lymphoma (HL) is still debated due to some remaining open issues. These involve the best choice of upfront chemotherapy, the duration and intensity of frontline treatment, the value of consolidation radiotherapy, the role of functional imaging in treatment planning and monitoring and the adequacy of risk stratification tools. These observations have led us to investigate whether intensification of front-line ABVD would be possible while maintaining acceptable levels of toxicity and improving the performance of the schedule. We designed a dose-dense three-weekly version of the ABVD regimen (six cycles), which was also dose intensified, in the first four cycles, by escalating doxorubicin dose from 50 to 70 mg/m2 per cycle, in the absence of consolidation RT. A series of 172 patients (advanced-stage=124, intermediate-stage=48) was studied. Apart from pre- and post-Tx staging, all patients also underwent 18F-FDG-PET imaging after the 2nd cycle (PET2); in case of pathologic uptake at PET2, a new scan was performed after the 4th cycle (PET4). Open-label phase study began I June 2004; specifications are contained in the published article (Russo F BHJ 166,1118,2014) focused on feasibility and toxicity in 82 patients. The demographics and clinical characteristics are in Tab1.
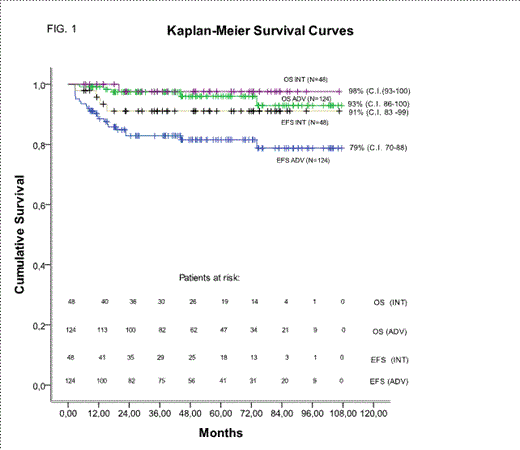
Ninety-five percent of patients completed the planned six cycles (median duration time=16.7 weeks). Median actual dose intensities were 20.94 (23.12 cycles1-4), 6.69, 3.96 and 245 mg/m2/week for doxorubicin, bleomycin, vinblastine and dacarbazine, respectively. This corresponded to a 66.9% (85.0%, cycles 1-4) increase in dose intensity for doxorubicin, (total dose 380 mg/m2) and of 32% for the other agents, over standard ABVD. Intensified ABVD was highly tolerated with low rates of hospitalization during treatment, a low incidence of G3/ G4 events, low post-treatment cardiac and pulmonary toxicities and a very low rate of gonadic toxicity. Only two cases of second cancer have been recorded. PET2 was negative in 87% of patients (85% ADV,92%INT). Remarkably a CR rate of 94% (93% ADV, 98% INT) was achieved and EFS and OS at 7-years were 85% (79%ADV, 91% INT) and 96% (93%ADV, 98%INT) respectively (Tab2, Fig1). At univariate analysis (factors: Age, sex, LDH, IPS,B-symptoms, mediastinal bulky disease (MBD), E-disease, PET2, stage IV) the predictive factors of low CR rate were IPS≥3 (CR 89% vs 97%, p=0.03), and PET2pos (CR 65% vs 99%p<0.001). At multivariate analysis PET2 positivity (p<0.001) was the only independent risk factor predictive of low CR rate. Kaplan-Meyer 7yr-EFS was 77 and 78% (Log Rank 1.1, p=0.28) in MBD and no-MBD subsets, respectively. 7yr-EFS was significantly better in patients with PET2neg (87% vs 52% , log rank 24.7, p<0.001) and in patients with IPS 0-2 (88% vs 71% log rank 4.9, p=0.02). At Cox regression analysis PET2 was the only independent factor predictive of Event Free Survival.
Intensified ABVD without radiotherapy seems to be adequately powered to exploit early disease chemo-sensitivity yielding high percentage of early negative PET2, high rate of response and survival, without new severe or critical short and long-term toxicities.
| TAB 1 . | . | Advanced-stage(n=124) . | Intermediate-stage (n=48) . | . |
|---|---|---|---|---|
| Characteristics | No. | % | No. | % |
| Age, years | ||||
| median | 32 | 29 | ||
| range | 15-77 | 15 - 62 | ||
| >45 years | 23 | 19 | 5 | 10 |
| Gender, male | 64 | 52 | 18 | 37 |
| Ann Arbor stage | ||||
| II | 32 | 26 | 48 | 100 |
| III | 40 | 32 | ||
| IV | 52 | 42 | ||
| B symptoms | 88 | 71 | 15 | 31 |
| Mediastinal bulk | 56 | 45 | 9 | 19 |
| Extramediastinal bulk ≥10cm | 6 | 5 | 2 | 4 |
| ≥ 3 nodal areas involved | 99 | 80 | 36 | 75 |
| Spleen involvement | 23 | 19 | ||
| Extranodal (stage II-III) | 8 | 6 | 2 | 4 |
| Extranodal (stage IV ) | 51 | 41 | ||
| ESR (>50 mm/h) | 60 | 48 | 15 | 31 |
| IPS ≥ 3 | 61 | 49 | 4 | 8 |
| LDH ratio>1 | 51 | 41 | 12 | 25 |
| Histologic subtype | ||||
| Nodular sclerosis | 102 | 82 | 40 | 83 |
| Lymphocyte rich | 10 | 8 | 5 | 10 |
| Mixed celluarity | 6 | 5 | 3 | 6 |
| NOS | 6 | 5 | 0 | 0 |
| TAB 1 . | . | Advanced-stage(n=124) . | Intermediate-stage (n=48) . | . |
|---|---|---|---|---|
| Characteristics | No. | % | No. | % |
| Age, years | ||||
| median | 32 | 29 | ||
| range | 15-77 | 15 - 62 | ||
| >45 years | 23 | 19 | 5 | 10 |
| Gender, male | 64 | 52 | 18 | 37 |
| Ann Arbor stage | ||||
| II | 32 | 26 | 48 | 100 |
| III | 40 | 32 | ||
| IV | 52 | 42 | ||
| B symptoms | 88 | 71 | 15 | 31 |
| Mediastinal bulk | 56 | 45 | 9 | 19 |
| Extramediastinal bulk ≥10cm | 6 | 5 | 2 | 4 |
| ≥ 3 nodal areas involved | 99 | 80 | 36 | 75 |
| Spleen involvement | 23 | 19 | ||
| Extranodal (stage II-III) | 8 | 6 | 2 | 4 |
| Extranodal (stage IV ) | 51 | 41 | ||
| ESR (>50 mm/h) | 60 | 48 | 15 | 31 |
| IPS ≥ 3 | 61 | 49 | 4 | 8 |
| LDH ratio>1 | 51 | 41 | 12 | 25 |
| Histologic subtype | ||||
| Nodular sclerosis | 102 | 82 | 40 | 83 |
| Lymphocyte rich | 10 | 8 | 5 | 10 |
| Mixed celluarity | 6 | 5 | 3 | 6 |
| NOS | 6 | 5 | 0 | 0 |
| . | Advanced stage . | Intermediate stage . | ||||
|---|---|---|---|---|---|---|
| Tab 2 | N | % | 95%C.I. | N | % | 95%C.I. |
| Final treatment response | 123 | 100 | 48 | 100 | ||
| CR | 115 | 93 | 88 - 97 | 47 | 98 | 94–100 |
| NearCR/PR | 6 | 4.9 | 1 | 2.1 | ||
| Progression | 2 | 1.6 | 0 | 0 | ||
| Cycle 2 PET | 124 | 100 | 48 | 100 | ||
| negative | 106 | 85 | 44 | 92 | ||
| positive | 18 | 15 | 4 | 8 | ||
| Cycle 4 PET | 18 | 100 | 4 | 100 | ||
| negative | 11 | 61 | 3 | 75 | ||
| positive | 7 | 39 | 1 | 25 | ||
| Events | 22 | 21 | 4 | 8 | ||
| <CR | 6 | 5 | 1 | 2 | ||
| progression | 2 | 2 | 0 | 0 | ||
| early relapse 3-12 | 8 | 6 | 2 | 4 | ||
| late relapse>12 months | 3 | 2 | 1 | 2 | ||
| 2nd tumor | 2 | 2 | 0 | 0 | ||
| 1st line treatment-related death | 1 | 1 | 0 | 0 | ||
| 1-yr EFS | 85 | 83-94 | 95 | 94–100 | ||
| 2-yr EFS | 83 | 76-90 | 91 | 83-99 | ||
| 4-yr EFS | 82 | 74-89 | ||||
| 7-yr EFS | 79 | 70-88 | 91 | 83-99 | ||
| 7-yr OS | 93 | 86-100 | 97 | 93–100 | ||
| . | Advanced stage . | Intermediate stage . | ||||
|---|---|---|---|---|---|---|
| Tab 2 | N | % | 95%C.I. | N | % | 95%C.I. |
| Final treatment response | 123 | 100 | 48 | 100 | ||
| CR | 115 | 93 | 88 - 97 | 47 | 98 | 94–100 |
| NearCR/PR | 6 | 4.9 | 1 | 2.1 | ||
| Progression | 2 | 1.6 | 0 | 0 | ||
| Cycle 2 PET | 124 | 100 | 48 | 100 | ||
| negative | 106 | 85 | 44 | 92 | ||
| positive | 18 | 15 | 4 | 8 | ||
| Cycle 4 PET | 18 | 100 | 4 | 100 | ||
| negative | 11 | 61 | 3 | 75 | ||
| positive | 7 | 39 | 1 | 25 | ||
| Events | 22 | 21 | 4 | 8 | ||
| <CR | 6 | 5 | 1 | 2 | ||
| progression | 2 | 2 | 0 | 0 | ||
| early relapse 3-12 | 8 | 6 | 2 | 4 | ||
| late relapse>12 months | 3 | 2 | 1 | 2 | ||
| 2nd tumor | 2 | 2 | 0 | 0 | ||
| 1st line treatment-related death | 1 | 1 | 0 | 0 | ||
| 1-yr EFS | 85 | 83-94 | 95 | 94–100 | ||
| 2-yr EFS | 83 | 76-90 | 91 | 83-99 | ||
| 4-yr EFS | 82 | 74-89 | ||||
| 7-yr EFS | 79 | 70-88 | 91 | 83-99 | ||
| 7-yr OS | 93 | 86-100 | 97 | 93–100 | ||
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.