Abstract
INTRODUCTION: Secondary central nervous system (CNS) dissemination is a lethal event in patients (pts) with aggressive lymphomas. A few studies focused on the treatment of this condition are available, confirming the dismal prognosis and the high rate of severe neurotoxicity in pts managed with radiotherapy-based induction. Thus, the most effective treatment for secondary CNS lymphoma remains an important, unmet clinical need. Herein, we report the final results of a multicenter phase II trial addressing a new treatment in pts with aggressive B-cell lymphoma and CNS involvement (NCT00801216). Experimental treatment is based on the encouraging experiences with high doses of antimetabolites in pts with primary CNS lymphoma (Ferreri et al. Lancet 2009), and with high-dose sequential chemotherapy combined with rituximab (R-HDS) and supported by autologous stem cell transplant (ASCT) in pts with relapsed aggressive B-cell lymphoma (Tarella et al. JCO 2008).
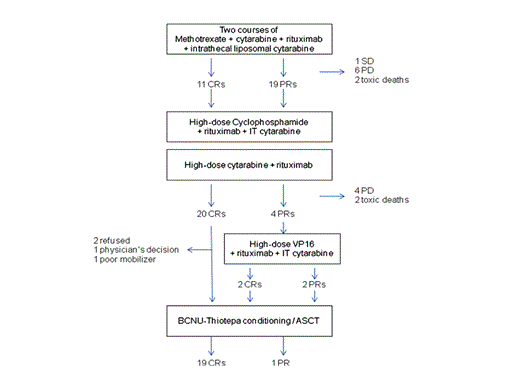
METHODS: Selection criteria were: 1) histological diagnosis of diffuse large B-cell lymphoma (DLBCL), mantle cell lymphoma blastoid variant (MCLb) or grade-3 follicular lymphoma (FL); 2) secondary CNS involvement at diagnosis or relapse; 3) age 18-70 years; 4) ECOG PS ≤3; 5) absence of HIV infection; 6) adequate organ functions. Pts with primary CNS lymphoma (exclusive CNS disease at diagnosis) were excluded. Experimental treatment consisted of an induction phase with 2 courses of methotrexate 3.5 g/m2 d1 + cytarabine 2 g/m2 x2/d d2-3, followed by an intensification phase with R-HDS (cyclophosphamide 7 g/m2 d1; cytarabine 2 g/m2 x2/d d22-25; pts with residual extra-CNS disease received also etoposide 2 g/m2 d43) and a consolidation phase with BCNU-thiotepa conditioning + ASCT (figure). Treatment included 8 doses of rituximab and 4 of intrathecal liposomal cytarabine. The primary endpoint was 2-year PFS; the planned accrual was 38 pts.
RESULTS: 39 pts were registered (age 32-70 ys, median 59; M/F ratio 1.5): 33 had DLBCL, 3 MCLb, 3 FL. CNS disease (brain 21, meninges 5, spinal cord 2, multiple 11) was detected at diagnosis in 16 pts (all with extra-CNS disease) and at relapse in 23 (8 with extra-CNS disease). The median TTP from the previous treatment line was 3 months (range 0-77 months).
Thirty-four pts completed the induction phase; 73 (93%) of 78 planned courses were actually delivered; G4 neutropenia, thrombocytopenia and anemia were recorded in 77%, 63% and 5% of courses, G3-4 febrile neutropenia in 16%, CMV reactivation in 3 pts. Transient G4 transaminases increase (3% of courses) was the only G4 non-hematological toxicity. Drugs dose reduction was indicated in 3 pts. Response after induction phase was complete in 11 pts and partial in 19 (ORR= 77%; 95%CI=64-90%).
Thirty pts were referred to intensification phase (figure); 58 (97%) of 60 planned courses were actually delivered; G4 neutropenia, thrombocytopenia and anemia were recorded in 67%, 60% and 7% of courses, G3-4 febrile neutropenia in 22%, G4 sepsis in 8%. CMV reactivation was diagnosed in 4 pts, pulmonary aspergillosis in 1. No cases of G4 extra-hematological toxicity were recorded. Response after intensification phase (before conditioning) was complete in 22 pts and partial in 2 (ORR= 62%; 95%CI=47-77%). ASCs were collected in 21/22 (95%) pts (median 9.5 x 106/kg; range 6-19); 20 pts underwent ASCT. Response at the end of the whole program was complete in 23 pts and partial in 1 (ORR= 62%, 95%CI= 47-77%), 1 pt had SD, 10 experienced PD (all in the CNS), and 4 died of toxicity (sepsis 2, stroke, acute tracheal obstruction). Importantly, no pt was irradiated to the brain to achieve lymphoma remission, and no evidence of neurotoxicity was recorded in pts with a survival longer than 3 years. One pt was referred to sibling-donor transplant due to sMDS, and is alive and NED at 91 months of follow-up.
At a median follow-up of 42 months, 16 pts remain relapse-free, with a 2-yr PFS of 42±8%. Sixteen pts are alive, with a 2-yr OS of 42±8%; 2-yr OS of transplanted pts was 72±11%. Extra-CNS and/or meningeal disease did not affect outcome, and survival was similar in both pts treated at presentation or at relapse.
CONCLUSION: This radiotherapy-free combination of high doses of antimetabolites, R-HDS and ASCT is feasible and effective in pts ≤70 ys with secondary CNS lymphoma. Toxicity is usually haematological and manageable. Survival benefit is attainable also in pts with meningeal and/or concomitant extra-CNS disease.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.