Abstract
Introduction:Histologic transformation (HT) has been well characterized in follicular lymphoma (FL), and has historically carried a poor prognosis with median overall survival (OS) of 1.2-1.8 years (yrs) in the pre-rituximab era (Montoto et al, 2007; Al-Tourah et al 2008). Despite improved outcomes in the rituximab era (Link et al, 2013), HT still represents a major source of mortality and morbidity in these patients (pts). While there is emerging evidence that autologous stem cell transplant (ASCT) confers a survival advantage compared to chemotherapy with rituximab alone (Ban-Hoefen et al, 2013; Villa et al, 2013), many of these patients have contraindications such as advanced age and frailty precluding ASCT as an option. We report a strategy of radioimmunotherapy (RIT) consolidation after chemoimmunotherapy to address this unmet need.
Methods: Consecutive pts ≥ 18 yrs old, who received RIT as consolidation after chemoimmunotherapy for HT at the Wilmot Cancer Institute were identified who had been deemed ineligible for ASCT. Inclusion criteria were as follows: 1) clinical, composite or pathologic diagnosis of HT, and 2) receipt of RIT after response to rituximab and chemotherapy. HT was defined as biopsy confirmed diffuse large B cell or Burkitt-like lymphoma by the WHO classification ≥6 months following a biopsy establishing indolent lymphoma diagnosis (pathologic HT), or clinical evidence of transformation as defined in previous cohorts (Al-Tourah et al, 2008; Link et al, 2013) in the absence of biopsy confirmation (clinical HT). Composite diagnosis of HT was defined as concurrent indolent and large cell lymphoma. The primary endpoint was overall survival (OS). Progression free survival (PFS) was a secondary outcome. Kaplan-Meier survival estimation curves were generated using SAS (SAS Institute Inc., Cary, NC, USA), and p values were generated using the log rank test.
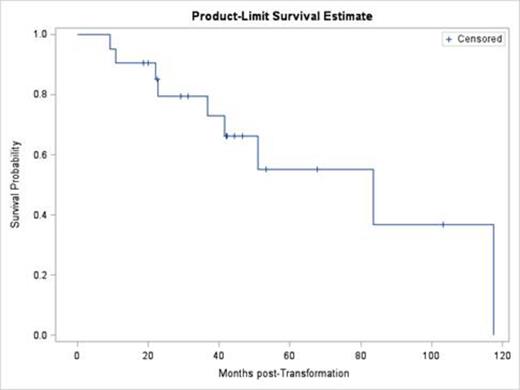
Results: 21 consecutive pts who had a clinical, composite, or pathologic HT diagnosis were included in this analysis. All of these pts responded to rituximab and chemotherapy (CHOP like n=18, other n=3), and were treated with RIT for consolidation. The median age at the time of HT was 66 yrs (44-90). Nine pts received I-131 tositumomab and 12 received Y-90 ibritumomab tiuxetan. The median OS from HT was 6.9 yrs in the entire cohort with a median follow up of 3.5 yrs (Figure). There were no statistical differences in outcomes between the pathologic (n=11) and clinical/composite (n=10) HT pts (p=0.33). There was also no difference between those who received rituximab prior to HT (n=13), and those who did not (n=8; p=0.88). Twelve pts experienced grade 3-4 thrombocytopenia, which was the most common adverse event (AE). Nine deaths occurred. Disease-related death was the most common cause (n=4), followed by death unrelated to disease or treatment (n=3) and treatment-related death (n=2).
Kaplan-Meier estimate of OS after HT in pts receiving RIT after chemoimmunotherapy (n=21)
Kaplan-Meier estimate of OS after HT in pts receiving RIT after chemoimmunotherapy (n=21)
Conclusions: We report the use of RIT as consolidation of response to chemoimmunotherapy in pts with HT. This represents one of the largest series focused on pts with HT who were deemed unfit for ASCT. The RIT was well tolerated in this analysis with only 2 treatment related deaths, one of which was secondary to therapy related acute myeloid leukemia. Our observed median OS compares favorably to published reports of pts with HT who are younger and able to tolerate more aggressive therapeutic options. Consolidative RIT should be considered as a treatment approach in this vulnerable population of pts, and can serve as a platform for the incorporation of novel agents into prospective clinical trials.
Barr:Pharmacyclics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.