Abstract
Introduction:
Constitutive hyperactivation of the STAT3 and STAT5B transcription factors is often observed in cancer. Lately, activating STAT3 mutations have been identified in hematological malignancies including large granular lymphocytic (LGL) leukemia (prevalence 40%), aplastic anemia (7%) and CD30+ T-cell lymphoma (17%). Furthermore, recent studies highlight the importance of STAT5B mutations in the pathogenesis and prognosis of T-cell malignancies such as T-cell prolymphocytic leukemia (36%), T-cell acute lymphoblastic leukemia (8%) and hepatosplenic T-cell lymphoma (33%). While STAT3 and STAT5B mutations lead to constitutive STAT3/STAT5B signaling, several other known gene mutations and mechanisms may also cause JAK/STAT-pathway activation. These findings indicate that inhibiting the JAK/STAT pathway with targeted drugs could be used as a treatment option. Here, we aimed to identify drugs that inhibit STAT3 or STAT5B function and determine if mutant STAT3/5B and wild type STAT3/5B cells respond differently to the tested drugs. In addition, we wished to ascertain if STAT3 inhibition is sufficient to induce apoptosis in patient derived LGL cells with constitutively active STAT3.
Methods:
High-throughput drug sensitivity testing was performed with a compound collection containing over 300 approved and investigational oncology drugs including many kinase inhibitors (such as those targeting JAK, SRC, VEGFR, mTOR, MEK, and CHK) and small molecule STAT3 inhibitors (Stattic, LLL12, Sta-21). All drugs were tested in 5-8 different concentrations over a 10,000-fold concentration range. Mutant STAT3 (Y640F) and mutant STAT5B (N642H) transformed Ba/F3 cells as well as HEK293 cells containing a STAT5 (pGL4.52[luc2P/STAT5 RE/Hygro]) or STAT3 specific luciferase reporter gene element (HEK-SIE) were used in the screens. In addition, drug sensitivities of five LGL leukemia patient samples were also assessed. Primary patient cells and the Ba/F3 cells were incubated in 384-well plates for three days with the drugs after which cell viability was measured with CellTiter-Glo. STAT3/5B induced luciferase activity in the HEK cells was analyzed after the cells were incubated for 6 or 24 hours with the drugs using the ONE-Glo luciferase assay system.
Results:
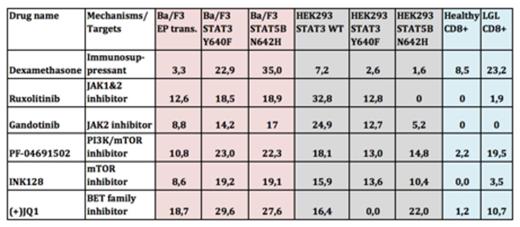
A significant decrease in luciferase activity was detected in STAT3 mutant Y640F, STAT5B mutant N642H and wild type STAT3 transfected HEK-SIE cells in the presence of PI3K/mTOR inhibitors such as PF-04691502 and INK128. In addition, PI3K/mTOR inhibitors significantly decreased the viability of mutant STAT3 and STAT5B transformed Ba/F3 cells compared to wild type cells. Interestingly, JAK inhibitors (e.g. ruxolitinib, gandotinib) did not inhibit mutant STAT3 activity in the HEK-SIE cells, whereas IL6-induced wild type STAT3 activity was completely blocked. A BET family inhibitor (JQ1+) and glucocorticoids (e.g. dexamethasone, methylprednisolone) showed specific and strong cytotoxicity to mutant STAT3 and STAT5B transformed Ba/F3 cells. Although JQ1+ inhibited luciferase activity of STAT5B N642H cells, no effect on the luciferase activity of STAT3 Y640F transfected HEK cells was detected, suggesting that JQ1+ may have a direct effect on mutant STAT5B function while the effect on mutant STAT3 transformed cells may be indirect. Cells from LGL leukemia patients showed high sensitivity against glucocorticoids, the histone deacetylase inhibitor quisinostat, JQ1+ and PF-04691502 when compared to healthy CD8+ T-cells. However, no increase in apoptosis was observed with JAK or other mTOR inhibitors.
Conclusions:
Our results suggest that JAK inhibitors lack efficacy in STAT3 mutated diseases. However, our ex vivo and in vitro drug screens highlight some other promising agents including PF-04691502 (PI3K/mTOR inhibitor) and JQ1+ (BET family inhibitor) that inhibited mutant STAT5B and STAT3 activity in the cell line models and were effective against primary LGL patient cells. Additional experiments are ongoing to determine how these drugs function to block STAT signaling and induce cell death. As the STAT3 and STAT5 pathways are activated in many other cancer types as well, the results may be applicable to a variety of different malignancies.
Table 1. Drug sensitivity scores of the different cell line models and patient samples. DSS value range 0-50. (0 = no drug response with any conc., 50 = maximal drug response with every conc.)
Mustjoki:Bristol-Myers Squibb: Honoraria, Research Funding; Novartis: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.