Abstract
Abstract
Introduction
The introduction of the tyrosine kinase inhibitor (TKI) imatinib has dramatically improved outcomes in chronic myeloid leukemia (CML) patients. A previous report from the DASISION study demonstrated remarkable improvement in treatment responses with dasatinib compared with imatinib. Regarding outcomes in chronic-phase CML (CML-CP) patients, the significance of an early treatment response was emphasized; the treatment goal primarily focused on molecular responses, i.e., major molecular response (MMR) and complete molecular response (CMR). However, although molecular response criteria seemed promising, the clinical entities such as the BCR-ABL transcript level at diagnosis varied in each patient. Recently, tumor reduction velocity was proposed as a useful predictor of patient outcomes. We therefore hypothesize that the halving time may sensibly predict molecular responses in dasatinib-treated patients. We conducted an open-label, multicenter, prospective "D-First" study (ClinicalTrials.gov; NCT01464411) and observed molecular responses of newly diagnosed CML-CP patients treated with dasatinib (100 mg QD).
Methods
Fifty-two newly diagnosed CML-CP patients were enrolled between June 2011 and June 2012. The primary endpoint of our study was the CMR rate by 18 months; therefore, all patients were followed-up for at least 18 months. To assess molecular responses, BCR-ABL transcripts were quantified using RQ-PCR. BCR-ABL transcripts were analyzed at diagnosis and 1, 3, 6, 9, 12, 15, and 18 months after initiating dasatinib treatment. BCR-ABL transcripts were measured as described previously (Yoshida C et al. Int J Clin Oncol. 2012;17:584). MMR was defined as <0.1% of BCR-ABLIS. CMR was defined as an undetectable BCR-ABL transcript level (<50 copies/µg RNA), which was equivalent to 4.16-log reduction. The rate of decline in BCR-ABL transcript was evaluated by the halving time for BCR-ABL transcripts, which was expressed using the following formula: Halving time = A x Log10 2/log10 (B/C), where A = the actual treatment time in days from the day of initial BCR-ABL transcript evaluation before treatment to BCR-ABL transcript evaluation at 3 months; B = BCR-ABL transcript levels before treatment (copies/µg RNA); and C = BCR-ABL transcript levels at 3 months (copies/µg RNA). If dasatinib treatment was transiently discontinued within the period, that interval was excluded from the actual treatment term (A).
Results
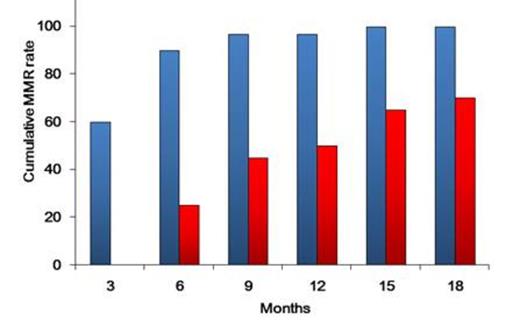
Patients enrolled included 31 men and 21 women with a median age at diagnosis of 50 years (range, 23–82 years). Of these, 51 received a once-daily 100-mg/day dasatinib dosage; 1 began with a50 mg/day dosage that increased to 100 mg/day 3 days later. Regarding molecular responses, 46 of 51 (90%) patients achieved ≤10% BCR-ABLIS by 3 months, and 46 of 51 (90%) reached ≤1% by 6 months. Further, 39 of 51 (76%) reached MMR by 12 months. Regarding CMR, 4, 15, and 26 of 51 evaluable patients obtained CMR by 3, 6, and 12 months, respectively. The CMR rate by 18 months was 59% (30/51). The receiver operating characteristic curve indicated a close correlation with the highest Youden's index at the halving time of 12.5 days. When patients were divided into 2 groups: longer halving time (>12.5 days) and shorter halving time (≤12.5 days), the shorter halving time group exhibited MMR rates of 97 % by 12 months and 100% by 15 months. In contrast, the longer halving time group had the significantly lower MMR rate of 50% by 12 months (P =0.0002; Figure 1). We also investigated the significance of the Sokal score on MMR and CMR achievement and found no predictive ability for either MMR or CMR achievement.
Conclusions
Our study demonstrated high efficacy for dasatinib treatment in newly diagnosed Japanese CML-CP patients and showed that CML-CP patients treated with dasatinib can be stratified according to the early treatment response as evaluated by the halving time for BCR-ABL transcripts.
Iriyama:Novartis: Honoraria; Kyowa Hakko Kirin: Honoraria. Yoshida:Bristol-Myers Squibb: Honoraria; Novartis: Honoraria; Otsuka Pharmaceutical: Research Funding. Ohyashiki:Bristol: Honoraria, Research Funding. Morita:Bristol: Honoraria. Sakamaki:Bristol: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.