Abstract
INTRODUCTION: Suboptimal adherence to medication is a serious issue in the management of chronic myeloid leukemia (CML) and contributes to worse clinical outcomes for patients. (Noens L, et al. Blood. 2009;113(22):5401-11.) The objective of this analysis is to evaluate adherence to treatment of patients diagnosed with CML and exposed to the tyrosine-kinase inhibitors (TKIs) imatinib, dasatinib or nilotinib and to analyze CML-related hospitalizations costs across adherence levels.
METHODS: De-identified data from hospital databases and haematology files from 4 Italian sites were combined to select adult patients diagnosed with CML, newly treated with imatinib, dasatinib, or nilotinib from July 2009 until June 2012 (inclusion period). Newly treated patients were identified by the absence of previous prescriptions. Demographics and clinical characteristics were assessed at the date of the first prescription within the inclusion period. Adherence to TKIs was measured as proportion of days covered (PDC) over a 180-day period. (Wu EQ, et al. Curr Med Res Opin. 2010;26(12):2861-9.) PDC was computed as the actual number of days of TKI therapy supplied divided by 180, and multiplied by 100. Days' supply was calculated based on quantity dispensed and dosage unit per day prescribed to patients (a dose of 100 mg for dasatinib, 400 mg for imatinib and 800 mg for nilotinib). PDC (and, therefore, adherence) was categorized using standard thresholds: ≥80% (fully adherent'), 40%–79% (partially adherent'), and <40% (non-adherent'). (Rasmussen JN, et al. JAMA. 2007;297(2):177-86.) The average daily dose was also calculated dividing the total dose dispensed by the number of days of TKI therapy supplied. A multivariable logistic regression model was used to estimate the relationship between TKI therapy and adherence, controlling for age, gender, Charlson comorbidity index, prior use of antihypertensives, drugs for acid-related disorders and anti-inflammatory drugs and previous hospital discharges for cancer other than CML. Leukemia-related hospitalizations were also collected. The ethics committees for the local health units approved the study.
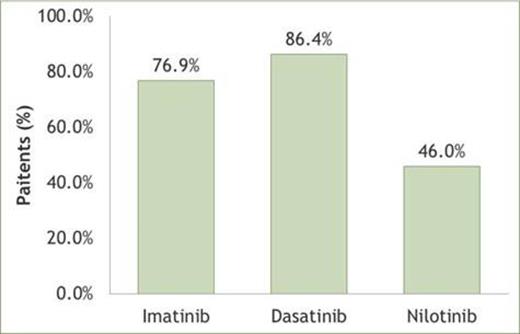
RESULTS: We identified 88 CML newly treated imatinib patients, 59 newly treated dasatinib patients and 50 newly treated nilotinib patients. Age, gender, Charlson comorbidity index and the prior use of the drug treatments listed above were similar at baseline. Dasatinib patients had fewer previous hospital discharges for cancer (P<0.001). As indicated in Figure 1, dasatinib newly treated patients were more adherent than imatinib- and nilotinib-naïve patients (86.4% vs 76.9% and 46.0% respectively; P<0.001).
Percentage of adherent patients (PDC≥80%) across the TKI therapy groups.
The mean daily dose calculated on the basis of the adherence was 377 mg for imatinib, 97 mg for dasatinib and 676 mg for nilotinib.
Comparing with dasatinib, multivariate logistic regression found significantly lower rates of adherence for imatinib (P=0.026) and for nilotinib (P<0.001), odds ratios (95% confidence intervals) resulted respectively of 0.24 (0.07-0.84) and of 0.10 (0.03-0.32). Independent of medication, higher adherence was associated with lower leukemia-related hospitalizations costs (the average cost per patient was 3,567 where PDC was 40%-79% and 554 where PDC≥80%). In addition, analyses were stratified by the presence or absence of previous hospital discharges for cancer. Among the 7 patients with previous hospital discharges for cancer, the average cost per patient was 6,935 where PDC was 40%-79% and 983 where PDC≥80%; while among the 190 patients with no previous hospitalizations for cancer, the average cost per patient was 536 where PDC was 40%-79% and 207 where PDC≥80%, confirming our findings in both cohorts.
CONCLUSIONS: This study showed that CML patients newly treated with imatinib or nilotinib were significantly less adherent than patients receiving dasatinib. On average, lower adherent patients had higher leukemia-related hospitalization costs. Further research is necessary to understand the relationship between adherence level and treatment response.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract