Abstract
Background
The promising development of perspectives in innovative therapeutic interventions in myelofibrosis (MF) makes the establishment of large collaborative networks ready to assure reliable comparability of cases and increasingly relevant data. This will provide assurance regarding efficiency and consistency in the development of clinical and epidemiological knowledge.
We report here the results of the pilot phase of ERNEST, whose acronym defines its purpose: European Registry for Myeloproliferative Neoplasms: towards a better understanding of Epidemiology, Survival and Treatment. This project promoted by the European LeukemiaNet (ELN) collaboration is coordinated by the Fondazione Mario Negri Sud (Italy) and supported by an unrestricted educational grant by Novartis.
Patients and Methods
In order to test the feasibility of a prospective epidemiological outcome-oriented registry across centers, expected to vary in the characteristics of their populations and care practices, a retrospective analysis was implemented based on a strictly pre-defined protocol. Patients with Primary Myelofibrosis (PMF), Post- Essential Thrombocythemia Myelofibrosis (PET-MF) and Post- Polycythemia Vera Myelofibrosis (PPV-MF) which were diagnosed in the participant centers between January 2001 and December 2012, with available follow-up information, were eligible for inclusion. Chi-square test and Mann-Whitney test were used to compare data at presentation by diagnosis for categorical and continuous variables respectively. Standard time-to-event methods were used for data analysis, including log-rank test, Kaplan-Meier survival graphs, and Cox proportional hazards models to calculate hazard ratios along with 95% confidence intervals (CI). Multivariable analysis was performed adjusting for unbalanced and relevant prognostic covariates.
Results
From February 2013 to May 2014, we received data of 1209 evaluable patients from 13 centers in 5 European countries (Italy, Germany, Spain, United Kingdom, Sweden): 61% were PMF, 20% PET-MF and 19% PPV-MF (median age: 66 years). 23%, 37% and 40% of diagnosis were performed between 2001-2004, 2005-2008 and 2009-2012 respectively.
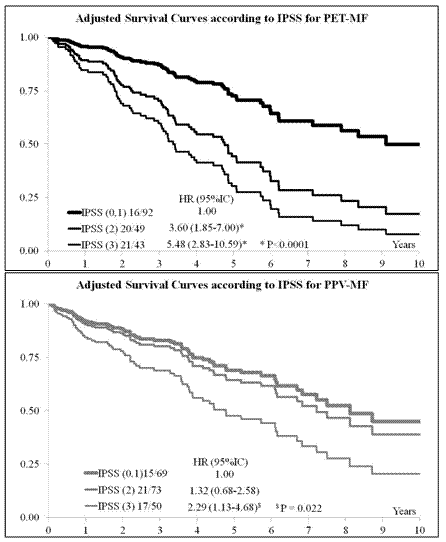
Variability was found for the presence of constitutional symptoms (from 43% in PET-MF to 49% in PPV-MF); an excess of splenomegaly emerged in PPV-MF cohort (84% vs 74% and 75% in PMF and PET-MF respectively). Mean value + SD of Hb, WBC and PLT were: 13.9 + 16.5 g/dl, 17.4 + 30.8 109/l, 372 + 316 109/l respectively with higher levels of Hb and WBC in PPV-MF patients than PET-MF and PMF (p<0.001). No variability was seen for presence of peripheral blasts and cytogenetic abnormalities. During follow-up (median duration: 2 years) 405 patients (33.5%) died without any differences among diagnosis subtypes. Leukemic transformation was experienced by 8% of the whole cohort (9% in PMF, 7% in PET-MF and 8%in PPV-MF). A multivariable’s Cox analysis was performed on the whole cohort including sex, diagnosis and International Prognostic Scoring System (IPSS) as covariates of interest. Besides male sex [Hazard Ratio (HR) 1.49 (95% CI (1.21-1.83), p<0.001] the prognostic significance of IPSS was confirmed with an HR 2.19 [(95% CI (1.64-2.92), p<0.001] for IPSS 2, and HR 4.20 [(95% CI (3.20-5.53), p<0.001] for IPSS >3, as compared with the reference category of patients with IPSS 0-1. As shown in Figure 1, an exploratory analysis documented different patterns of predictivity when the analysis was stratified according to the diagnosis subtypes. The determinants of the prognostic value of IPSS in PPV-MF vs PET-MF (and PMF, data not shown) would certainly deserve fully adjusted analysis in prospective well defined cohorts of patients.
Conclusions
The intensive quality control needed to assure the reliability, representativeness and the comparability of the data across international centers with expertise in this field confirms both the interest, but also the challenge of a cooperative epidemiological effort capable of representing a knowledge producing shared resource in the area of MF and other rare disease. Based also on the methodological and operational challenge resulting from this pilot study, a prospective study has been activated starting on September 2013.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.