Abstract
Background: Epstein-Barr virus (EBV) is a ubiquitous γ-herpesvirus that infects more than 90% of the world population. Following primary infection in the oropharynx, EBV remains latently present in B cells. Asymptomatic EBV reactivations periodically occur in oral mucosa-associated lymphoid tissues in healthy carriers. B cells infected by EBV are suspected to be involved in the etiology of various human lymphoma. Previously, EBV reactivation has been diagnosed by serologic IgG, IgM, IgA profiles that suggested virus replication. However, serologic responses, in immunocompromised subjects, like patients with chronic lymphocytic leukemia (CLL), do not necessarily indicate ongoing replicative activity. CLL is the most frequent form of leukemia in adults in western countries. Despite by definition chronic character, CLL is characterized by marked heterogeneity. Potential involvement of EBV in the pathogenesis of CLL is still to be explain. Latent EBV infection in healthy carriers is controlled by a cell-mediated immune response that is impaired in CLL patients and might result in poor control of EBV infection. Since EBV may activate B cells, stimulate their proliferation and inhibit their apoptosis, we hypothesize that it could contribute to unfavorable clinical course of CLL and may be one of reason of its heterogeneity.
Objective: The aim of this study was to determine whether EBV-DNA load in the peripheral blood mononuclear cells (PBMCs) of CLL patients may influence heterogeneity in clinical course of the disease. We analyzed the association between EBV-DNA load in PBMC and stage of the disease, adverse prognostic factors (including CD38 and ZAP-70 expression and cytogenetic abnormalities) and clinical outcome.
Material and methods: Peripheral blood samples were obtained from 115 untreated CLL patients and 20 healthy individuals. Real-time PCR was used to quantitate EBV-DNA copy number in PBMCs (viral load). As the sensitivity of the system amounts to 10 copies per µl, all the samples with the EBV-DNA copy number below this detection threshold were considered EBV-negative. The immunophenotype of peripheral blood lymphocytes was analyzed by flow cytometer.
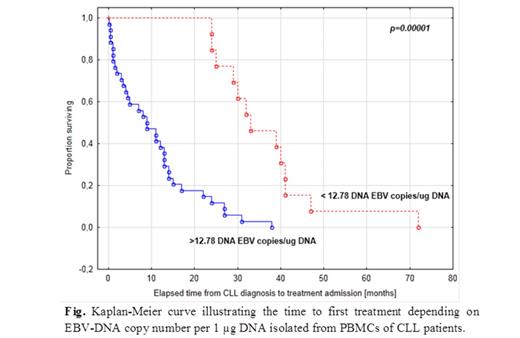
Results: Detectable EBV-DNA load in PBMCs was found in 62 out of 115 CLL patients (53.91%). The median EBV-DNA copy number in these group was 184 per 1 µg DNA (range: 12.79-8957.69). The EBV-DNA copy number per 1 µg DNA was significantly higher in patients who required the treatment (p<0.0001) than in untreated ones, in patients with lymphocyte count doubling time <12 months than in than patients with lymphocyte count doubling time >12 months (p<0.0001), in patients with hepatomegaly (p=0.023) and, splenomegaly (p=0.005) than in patients without organomegaly. It was also significantly higher in patients with CD38 (p<0.0001) and ZAP-70 expression (p=0.008) comparing to the patients CD38- and ZAP-70-, respectively, as well as in the patients with del (11q22.3) as comparing to the patients without this cytogenetic abnormality. Furthermore, the EBV-DNA copy number per 1 µg DNA showed significant positive correlation with the concentrations of LDH (r=0.531, p=0.00001) and beta-2-microglobulin (r=0.425, p=0.00057). The presence of more than 12.79 copies of EBV DNA in 1 µg DNA isolated from PBMCs was associated with reduced time to first treatment (p=0.00001), and lymphocyte count doubling-free survival (p<0.000001).
Conclusions: We proved for the first time, that higher of EBV-DNA copy number in PBMCs of patients with CLL is associated with adverse prognostic factors as well as more progressive clinical course of the disease expressed by reduced time to first treatment. These results suggest that higher of EBV-DNA copy number in peripheral blood mononuclear cells blood predicts poor clinical outcome of CLL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.