Abstract
Introduction: Multiple myeloma (MM) remains an incurable disease for most patients. Thus, sequential therapy when relapse or progression after first line is almost always needed. Immunomodulatory agents derived from thalidomide such as lenalidomide, has several antitumoral effects including anti-proliferative and immune-modulation by binding the intracellular protein cereblon. Lenalidomide has been approved for the treatment of relapsed or refractory MM in Europe and USA. The possibility of using biological predictors of response with this regimen could be promising. A group of genetic normal variations in DNA, mainly single nucleotide polymorphisms (SNPs), have been described in association with response to treatment in MM. A distinctive group of polymorphisms is constituted by SNPs in microRNAs (miRNA) processing machinery in miRNA precursor molecules and in miRNA binding sites, known as miRSNPs, that we have associated with response to autologous transplantation and bortezomib. The aim of this study was to ascertain the outcome of patients with relapsed myeloma treated with lenalidomide in a tertiary hospital as to investigate the prognostic impact of 9 miRSNPs located either in MM related miRNAs target genes or in miRNA biogenesis pathway proteins
Methods: One hundred and three patients (54M/49F; median age 65 years, range 30 to 86) with relapsed MM after at least one treatment regimen were treated from November 2003 to January 2014 with lenalidomide plus dexamethasone. Median follow-up for alive patients was 3 years. Patients had received a median number of 2 treatment lines (range 1 – 6), but with no lenalidomide-based regimens.
Genomic DNA was isolated from bone marrow slides using a commercial kit (Qiagen). The genes and SNPs evaluated in genomic DNA by allelic discrimination (TaqMan assays) were KRT81 (rs3660), FAM179b (rs1053667), MIR146A (rs2910164), MIR196A2 (rs11614913), MIR149 (rs2292832) and MIR423 (rs6505162) for miRNA target genes, and RAN (rs14035), TRBP (rs784567) and XPO5 (rs11077) for miRNA biogenesis pathway. These genes were selected based on their potential impact on prognosis in solid tumors in previous reports.
Results: Overall response (OR) was achieved in 55.4% of the patients (complete remission 6.8%, partial response 40.8% and minor response 7.8%), while 16 (15.5%) and 26 (25.2%) showed no response (NR) or progressive disease (PD), respectively. 4 patients (3.9%) died before response could be evaluated. The median progression-free survival (PFS) after lenalidomide therapy was 8.1 months (95% CI 7.3 to 8.9), with a median overall survival (OS) of 2.3 years (95% CI 1.5 to 3.2). OS was significantly shorter in those patients not reaching at least MR (1 vs. 3.7 years; p<0.0001). Six patients did not progress during the first 5 years of treatment: two have died for unrelated causes; two progressed biologically but no requiring further treatments and the remaining two are in continued response while on therapy. Only 8 responding patients (7.8%) in whom therapy with lenalidomide was discontinued received re-treatment with lenalidomide at progression.
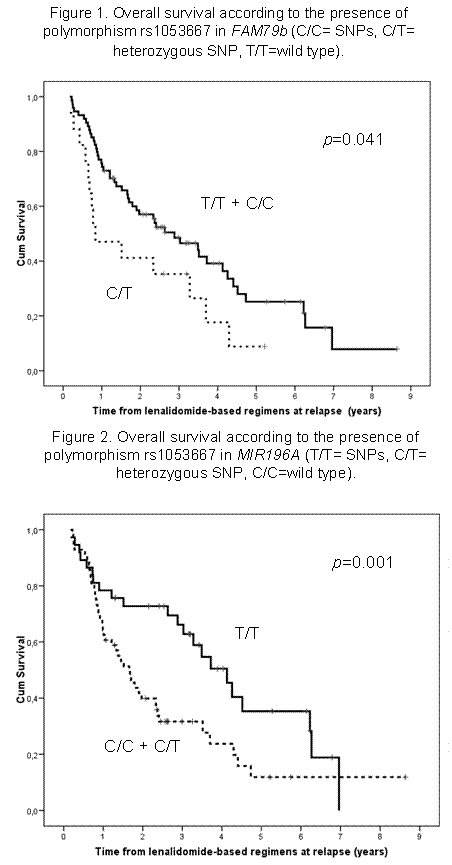
OS was significantly longer in patients with SNP in FAM179b (p=0.041) (Figure 1) and MIR196A2 (p=0.01) (Figure 2), with a trend for MIR149 (p=0.117). In the two first SNPs, there were also a significant impact in PFS after lenalidomide regimen (p=0.024 and p=0.018). Interestingly, these miRSNPs have not shown the same impact as previously reported when autologous transplantation or bortezomib are used, where XPO5 is the crucial gene. Other factors associated with longer OS included lenalidomide at first relapse vs. subsequent lines (p=0.002), IgG isotype (p=0.044), normal serum LDH (p=0.032) and a trend for creatinine lower than 2 mg/dL (p=0.08) at diagnosis. In a multivariate analysis for OS, only the use of lenalidomide at first relapse (HR 2, 95% IC 1.04-3.9; p=0.039) and SNP in MIR196A2 (HR 1.86, 95% IC 1.03-3.4; p=0.04) remained at significant level.
Conclusions: Lenalidomide plus dexamethasone is a very active regimen when used in daily clinical practice, particularly at the time of first relapse. A small subgroup of patients can remain disease-free for more than 5 years. Some SNPs in miRNA target genes (FAM179b and, particularly, MIR196A) could be useful as potential predictors of response.
Fernández de Larrea:Celgene: Consultancy, Honoraria. Cibeira:Celgene: Consultancy, Honoraria. Rosiñol:Janssen: Honoraria; Celgene: Honoraria. Jiménez:Janssen: Honoraria. Bladé:Janssen: Grant support Other, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Grant support, Grant support Other, Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.